ONLINE INQUIRY

Human Brain Microvascular Endothelial Cells
Cat.No.: CSC-C1503
Species: Human
Source: Brain
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Human brain microvascular endothelial cells (HBMECs) represent a specialized endothelial cell type located in cerebral micro-vessels which serves as an essential component of the blood-brain barrier. Brain endothelial barrier cells contain multiple tight junctions that create high trans-endothelial resistance to reduce paracellular flux and block substance entry from the bloodstream into the brain. The absence of fenestrae combined with reduced transcytosis levels for fluid-phase substances in HBMECs strengthens the barrier function compared to peripheral endothelial cells. These cells contain asymmetrically localized enzymes together with transporter systems that enable directional substance transport which maintains blood-brain barrier homeostasis.
The use of HBMECs is essential for drug permeability research because they provide a method to assess the ability of pharmaceuticals to penetrate the blood-brain barrier. The transport of neurotrophic factors by these cells helps to understand nervous system development and repair mechanisms. HBMVECs express adhesion molecules like ICAM-1 and VCAM-1 that control leukocyte movement and immune system activities. This feature makes them an important tool for studying immune-mediated brain injuries and neuroinflammation.
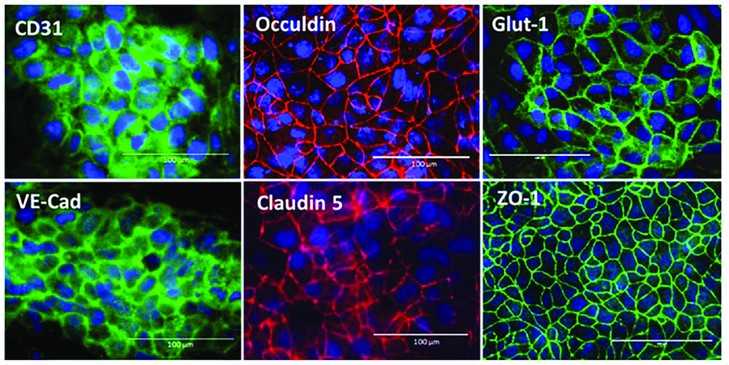 Fig. 1. Immunofluorescent staining of BMEC marker proteins, including adherence junction proteins (CD31, VE-cadherin), tight junction proteins (Occludin, claudin 5, ZO-1), and glucose transporter, Glut-1. Scale bars, 100 µm (Zhang W, Zhao X, et al., 2023).
Fig. 1. Immunofluorescent staining of BMEC marker proteins, including adherence junction proteins (CD31, VE-cadherin), tight junction proteins (Occludin, claudin 5, ZO-1), and glucose transporter, Glut-1. Scale bars, 100 µm (Zhang W, Zhao X, et al., 2023).
BMEC Microvasculatures Have a Better Endothelial Barrier Function than HUVEC Microvasculatures
Endothelial cells (ECs) specialized to organs have specific vascular features that are vital for ensuring function in the organ, particularly the BBB. While the literature has traditionally not included such important in vivo parameters as 3D structure and pericyte coverage, this has prevented simulations of genuine microvascular environments in vitro.
Uwamori's team employed a microfluidic platform to mimic such an environment by co-culturing human brain microvascular endothelial cells (BMECs) with mesenchymal stem cells (MSCs), allowing for evaluation of differences in vascular formation and barrier functions compared to human umbilical vein endothelial cells (HUVECs). They evaluated the endothelial barrier performance of 7-day-old microvasculatures derived from BMECs or HUVECs. Fluorescence dextran solution was introduced into microvascular networks for time-lapse imaging. We successfully introduced it into capillaries with diameters < 10 μm (Fig. 1A–C), and images confirmed the lumens were filled without leakage (Fig. 1B). They also plotted dextran diffusion from the vessels inside to the outside, and found sharp peaks around the wall of the capillary (Fig. 1D). Within 3 μm of the capillary wall, extravascular fluorescence increased over time (Fig. 1E). Permeability values for 70 kDa dextran ranged from 0.910 ± 0.405×10−7 cm/s in BMEC microvasculatures to 2.743 ± 0.550×10−7 cm/s in HUVEC microvasculatures (Fig. 1F), suggesting that the endothelial barrier function of BMEC microvasculatures was significantly more robust than that of HUVEC microvasculatures.
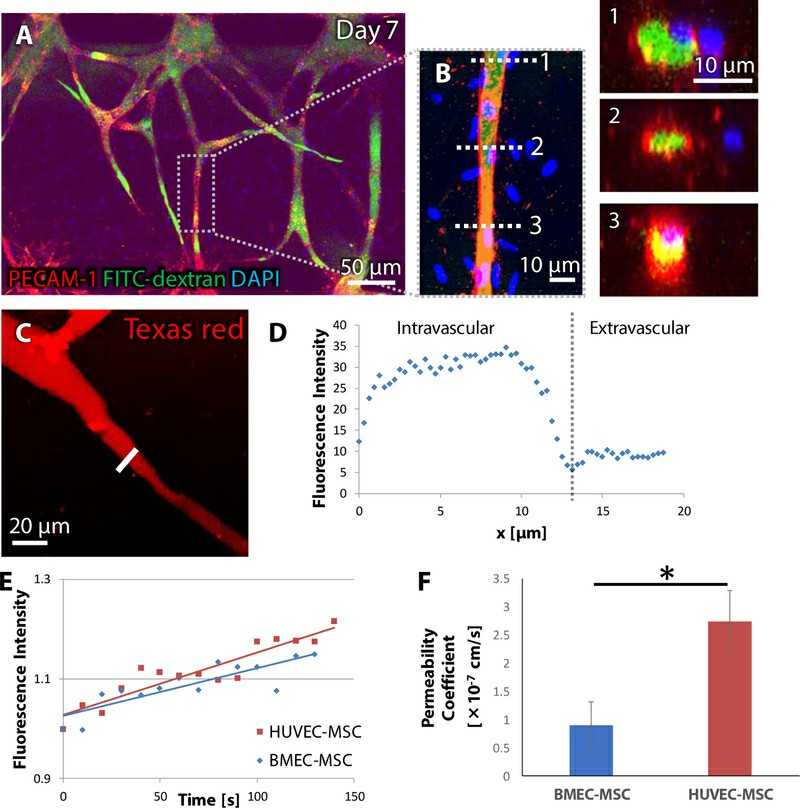 Fig. 1. Fluorescence dextran perfusion into constructed microvasculatures (Uwamori H, Ono Y, et al., 2020).
Fig. 1. Fluorescence dextran perfusion into constructed microvasculatures (Uwamori H, Ono Y, et al., 2020).
Des-Arg-9-BK Stimulates Secretion of Pro-Inflammatory Cytokines by hCMVEC Cells
Neuroinflammatory diseases like Alzheimer's and Parkinson's are marked by chronic inflammation and dysfunctional vascularity. These neuroinflammatory states are caused by activation of the Bradykinin 1 receptor (B1R), but no one understands how exactly B1R promotes inflammation and vascular dysfunction. Mugisho et al. assessed the impact of B1R stimulation via Des-Arg-9-BK on hCMVECs.
The levels of IL-6 and IL-8 in hCMVEC cells were used to assess endothelial inflammation at 4, 24, and 72 hours after treatment (Fig. 2). Des-Arg-9-BK treatment significantly boosted IL-6 at 24 hours and 72 hours, but not 4 hours. Expression of IL-8 increased dramatically at 72 hours, but not at earlier intervals. They also quantified the release of adhesion proteins ICAM-1 and VCAM-1, which significantly increased at 4, 24, and 72 hours after Des-Arg-9-BK administration. The levels of MCP-1 and VEGF were measured for leucocyte recruitment proteins (Fig. 2). Des-Arg-9-BK reduced MCP-1 by an order of magnitude at 24 hours but raised it at 72 hours. At 4 hours nothing was different. VEGF expression was significantly lower at 72 hours and did not change at 4 or 24 hours. These results indicated Des-Arg-9-BK stimulates secretion of pro-inflammatory cytokines by hCMVEC cells.
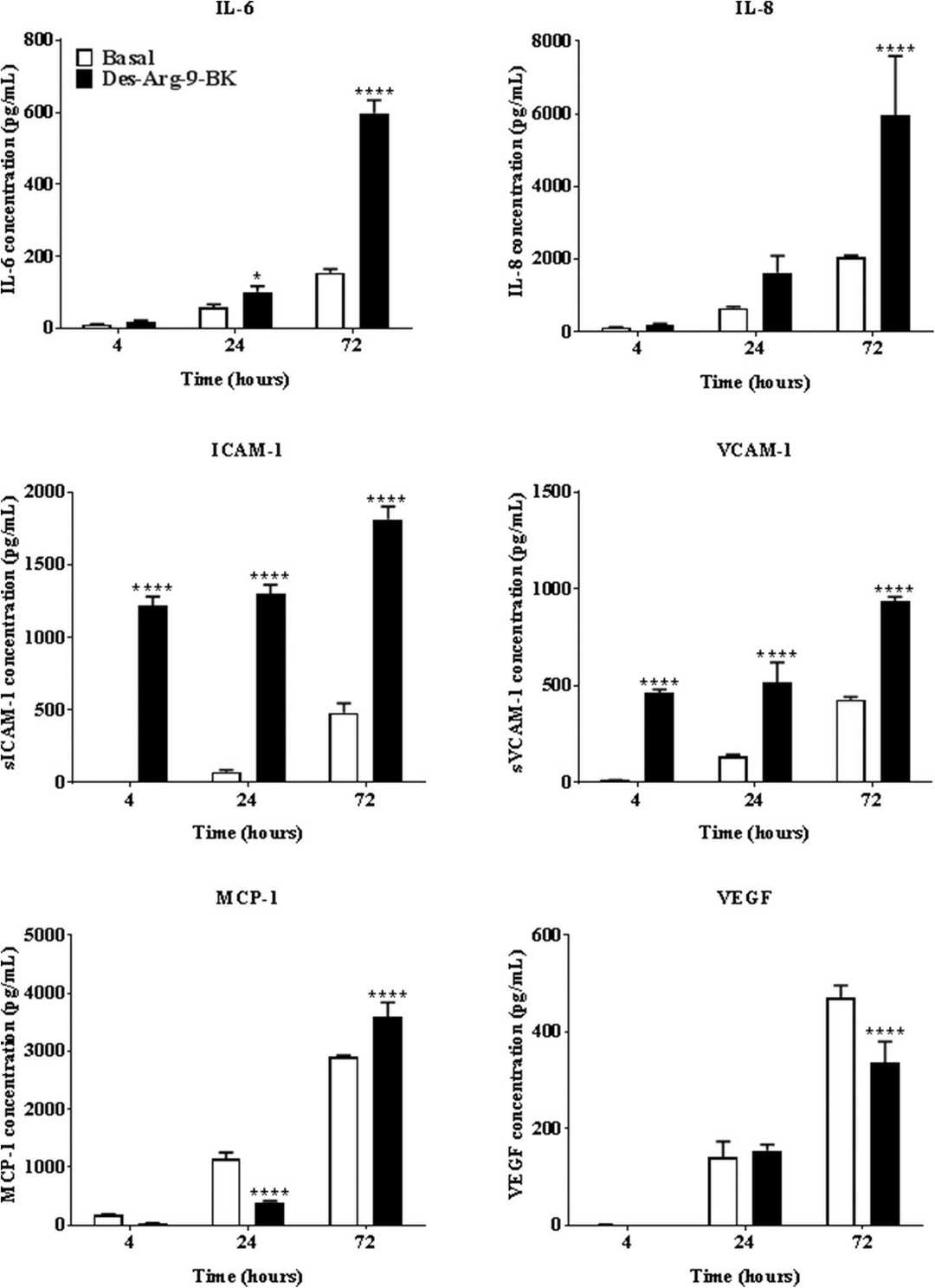 Fig. 2. Effect of Des-Arg-9-BK stimulation on the secretion of IL-6, IL-8, ICAM-1, VCAM-1, MCP-1 and VEGF by hCMVEC cultures (Mugisho OO, Robilliard LD, et al., 2019).
Fig. 2. Effect of Des-Arg-9-BK stimulation on the secretion of IL-6, IL-8, ICAM-1, VCAM-1, MCP-1 and VEGF by hCMVEC cultures (Mugisho OO, Robilliard LD, et al., 2019).
Yes, we can provide Immortalized Human Brain Microvascular Endothelial Cells.
Ask a Question
Write your own review