ONLINE INQUIRY

Human Splenic Fibroblasts (HSF)
Cat.No.: CSC-7709W
Species: Human
Source: Spleen
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human Splenic Fibroblasts (HSF) are cells isolated from human spleen tissue. As the largest secondary lymphoid organ in humans the spleen performs multiple immune functions which include blood pathogen filtration and abnormal cell removal as well as immune response regulation and hematopoietic activity. The spleen contains two main structures called red pulp and white pulp which serve different functions: red pulp removes old red blood cells and white pulp contains immune cells including B cells and T cells. Red pulp fibroblasts connect with red pulp macrophages to create a supportive reticular structure that ensures macrophage survival and functionality through CSF1 secretion. White pulp fibroblasts maintain lymphocyte microenvironments and control immune responses. They control the differentiation and functionality of B cells and T cells by utilizing the Notch signaling pathway. The spindle-shaped structure of splenic fibroblasts enables them to create a reticular network throughout the tissue.
HSF serves as a research model to understand how T cells and B cells interact in spleen immune functions. These models provide insight into the mechanisms of spleen-related disease pathogenesis including splenic fibrosis and support drug screening for immune regulation and fibrosis treatment.
Collagen I Level in HSF Is Down-Regulated During rPvTRAg23 Stimulation
The spleen is pivotal in Plasmodium infection, contributing to immune functions and filtration of aging cells, with splenic fibroblasts (SFs) playing a critical role. Type I collagen, secreted by SFs, forms part of the extracellular matrix crucial for immune response. During infections, the SFs become abnormally activated, impacting collagen I and allowing Plasmodium to evade immune responses.
Zhang's team evaluate the protein PvTRAg23, identified from spleen-dependent PvTRAgs of the Pv-fam-a gene family, to determine its effects on splenic fibroblasts and collagen I production. To evaluate PvTRAg23's effect on HSFs, cells were exposed to various concentrations of recombinant protein for 48 hours, with collagen I levels monitored. Increasing rPvTRAg23 concentrations reduced collagen I expression in HSFs, unlike the control rPvTRAg26 (Fig. 1A), showing that rPvTRAg23 affected the collagen I content in HSFs specifically and in a concentration-dependent manner. Similar decreases were seen in COL1A1 and COL1A2 mRNA levels (Fig. 1B). ELISA confirmed reduced collagen I in the HSF culture medium's supernatant after 48 hours of protein stimulation (Fig. 1C), indicating rPvTRAg23 reduced HSF collagen I production and secretion.
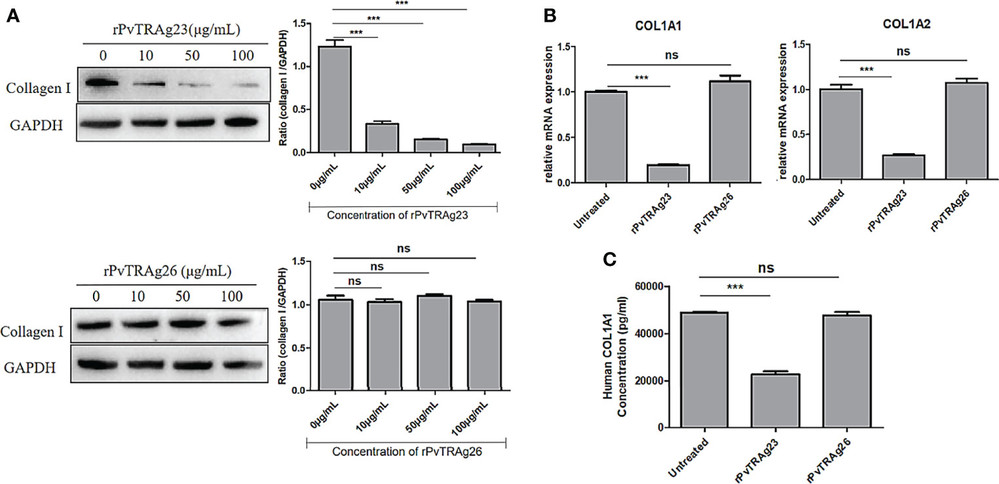 Fig. 1. The levels of collagen I secreted by HSFs after stimulated by recombinant proteins (Zhang H, Shen F, et al., 2022).
Fig. 1. The levels of collagen I secreted by HSFs after stimulated by recombinant proteins (Zhang H, Shen F, et al., 2022).
Human Splenic Fibroblasts Alter BCL-2 Dependence
T-cell ALL is a malignant disease, causing immature T cells to spread to multiple areas, including the spleen. ETP-ALL is a subtype with poor outcomes, relying heavily on BCL-2 for cell survival. While ABT-199 shows promise against it, acquired resistance remains a critical hurdle.
Grande et al. assessed if the human splenic microenvironment affects BCL-2 dependence. They used human splenic fibroblasts (HSF) to create an ex vivo coculture model (Fig. 2A). LOUCY cells cocultured with HSF for 48 hours showed reduced cytochrome c release after BAD BH3 peptide and ABT-199 treatment, indicating decreased BCL-2 dependence (Fig. 2A-B). This was linked to reduced sensitivity to ABT-199 but not to other BH3 mimetics (Fig. 2C, Fig. 3A-C). BCL-2 expression decreased, while BCL-XL increased, without impacting WEHI-539 sensitivity (Fig. 2D-E). Additionally, using a transwell migration assay, LOUCY cells migrated more toward HSF-conditioned media than HS-5 media, unlike typical T-ALL cells (Fig. 2F, Fig. 3D). Furthermore, they examined if HSF coculture with an ETP-ALL sample could change the BH3 profile. Although ETP-ALL samples are hard to grow in mice, ETP-5 showed high BCL-2 expression from patient-derived pediatric tumor models. ETP-5 was confirmed as BCL-2 dependent, being highly sensitive to BH3 mimetics ABT-199 and ABT-263 after 6 hours in vitro (Fig. 2G). Coculturing ETP-5 with HSF for 16 hours reduced the BAD BH3 peptide response by 20%, indicating decreased BCL-2 dependence (Fig. 2H). These results suggest HSF-conditioned media provides a strong migration signal and that HSF coculture can reduce BCL-2 dependence in LOUCY and ETP samples.
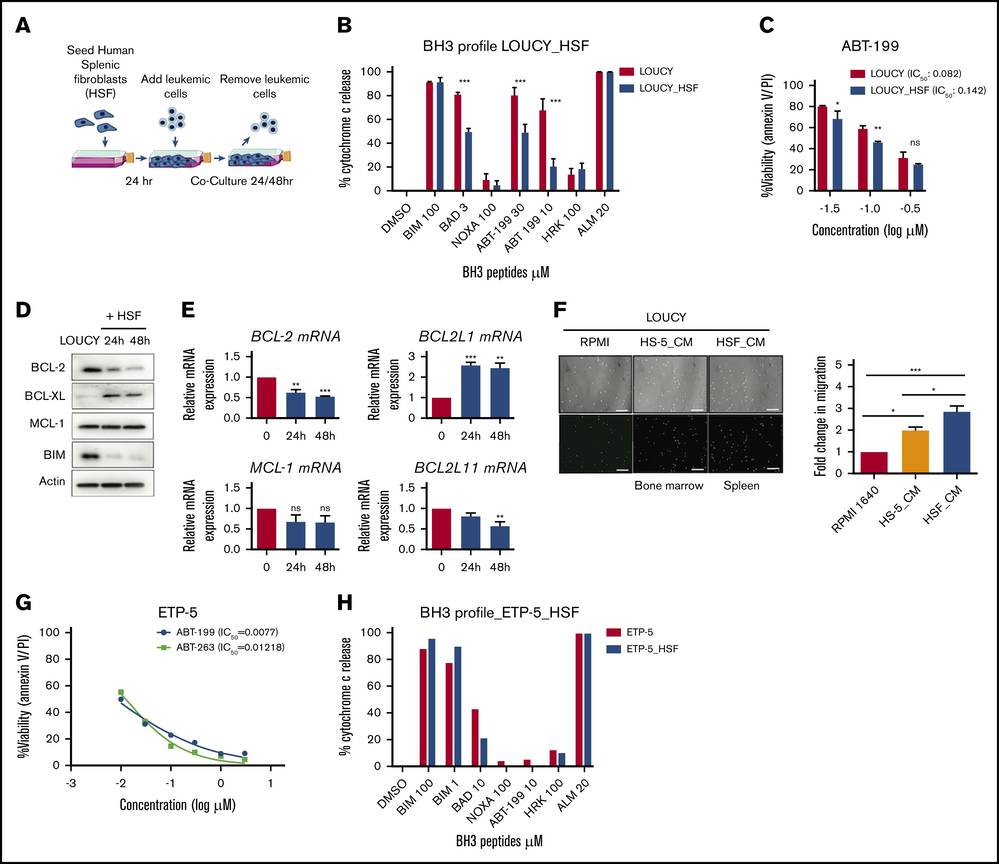 Fig. 2. Human splenic fibroblasts reduce BCL-2 dependence in ETP-ALL in vitro (Grande A D, Peirs S, et al., 2021).
Fig. 2. Human splenic fibroblasts reduce BCL-2 dependence in ETP-ALL in vitro (Grande A D, Peirs S, et al., 2021).
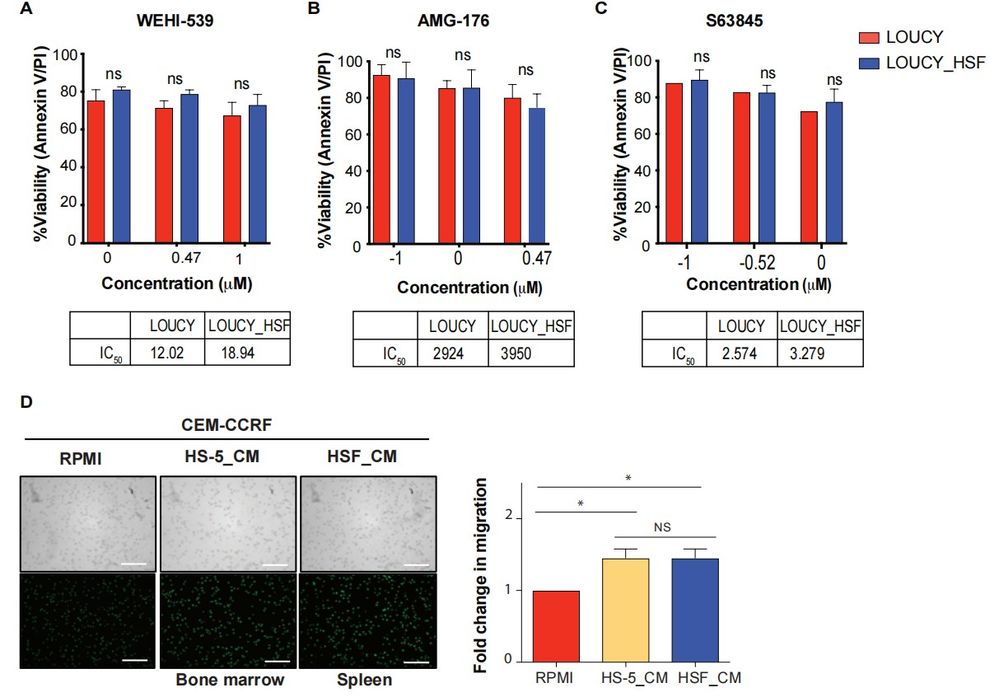 Fig. 3. Human splenic fibroblasts do not change sensitivity to BCL-XL or MCL-1 BH3 mimetics (Grande A D, Peirs S, et al., 2021).
Fig. 3. Human splenic fibroblasts do not change sensitivity to BCL-XL or MCL-1 BH3 mimetics (Grande A D, Peirs S, et al., 2021).
Ask a Question
Write your own review


