ONLINE INQUIRY

Human Small Intestinal Microvascular Endothelial Cells
Cat.No.: CSC-C8618W
Species: Human
Source: Small Intestine; Intestine
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human small intestinal microvascular endothelial cells are isolated from the microvascular wall of human small intestinal tissue. Small intestine between gastric pylorus and cecum is the largest organ of the digestive system, and its primary place of defecation and absorption. These cells are normally cobblestone-shaped, and feature prominent, dark-colored nuclei under the light microscope. They can be detected by the surface markers CD31 and von Willebrand factor (vWF). These cells are one of the primary cell types of the vessel walls that innervate the blood vessels, tightly packed so that a barrier keeps the artery's walls open so that blood-borne molecules don't get into the gut randomly. They also keep material from gastrointestinal tissues out of the bloodstream, thus mediating material flow between blood and gastrointestinal tissues. During inflammation, microvascular endothelial cells can capture and attach to on-circulating immune cells, which regulate inflammation.
In organ chip construction, co-culturing small intestinal microvascular endothelial cells with intestinal epithelial cells on the chip can simulate various physiological functions of the small intestine, such as the transport, absorption, and metabolism of nutrients. The fluid flow and peristalsis-like mechanical movements within the chip also allow for the study of dynamic intestinal processes. For instance, this organ chip model can be used to deeply investigate mechanisms and pathological processes related to small intestinal diseases, such as inflammatory bowel disease and intestinal obstruction.
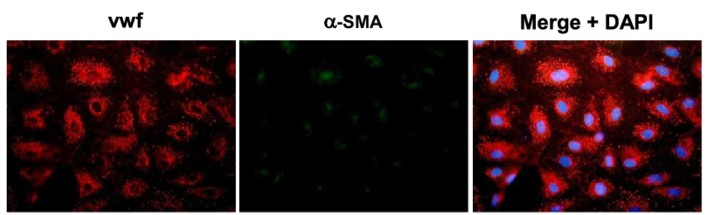 Fig. 1. Immunofluorescence images of human intestinal microvascular endothelial cells labeling with von Willebrand factor (VWF), a-smooth muscle actin (a-SMA) and DAPI (Mintet E, Rannou E, et al., 2015).
Fig. 1. Immunofluorescence images of human intestinal microvascular endothelial cells labeling with von Willebrand factor (VWF), a-smooth muscle actin (a-SMA) and DAPI (Mintet E, Rannou E, et al., 2015).
Development the Adult Duodenum Intestine-Chip Use Human Small Intestinal Microvascular Endothelial Cells
Orally administered drugs face bioavailability challenges mainly due to intestinal drug-drug interactions and enzyme activities. Traditional models, such as Caco-2, lack three-dimensional cytoarchitecture and accurate enzyme expression. Therefore, Kasendra's team combined intestinal organoids and Organs-on-Chips technology to develop a Duodenum Intestine-Chip. In summary, organoid cultures were established from biopsied crypts of healthy adults (Fig. 1A; top), and fragments were seeded on ECM-coated PDMS membranes in the chips (Fig. 1B; 3: epithelial tissue). Human small intestinal microvascular endothelial cells (HIMECs) (Fig. 1A; bottom) populated the PDMS membrane in the vascular channel (Fig. 1B; 4: endothelial cells). The Duodenum Intestine-Chip was continuously perfused with fresh medium, and cyclic mechanical strain (10% strain, 0.2 Hz) was applied to mimic intestinal peristalsis.
They evaluated the effects of mechanical stimulation on the phenotype of the intestinal cells using immunofluorescence and SEM for microvilli identification. Exposure to flow for 72 hours induced epithelial polarization and microvilli formation (Fig. 1 and Fig. 2), aligning with previous Caco-2 cell findings. Static cultures formed flat squamous cells with poor junctions (Fig. 1 and Fig. 2A) and sparse microvilli (Fig. 1 and Fig. 2B), while flow conditions yielded well-polarized cells with distinct junctions and dense microvilli. Continuous flow was crucial for epithelial maturation, and extended exposure to flow and strain led to 'villi-like structures' by day 6 (Fig. 1C). Confocal analysis showed confluent monolayers with defined tight and adherent junctions (Fig. 1D). These conditions improved intestinal permeability over time, indicated by low dextran permeability (Papp) in chips from different individuals (Fig. 1E). Overall, this data indicates that the human adult Duodenum Intestine-Chip supports the formation of a functional barrier with in vivo relevant cytoarchitecture, cell-cell interactions, and permeability parameters.
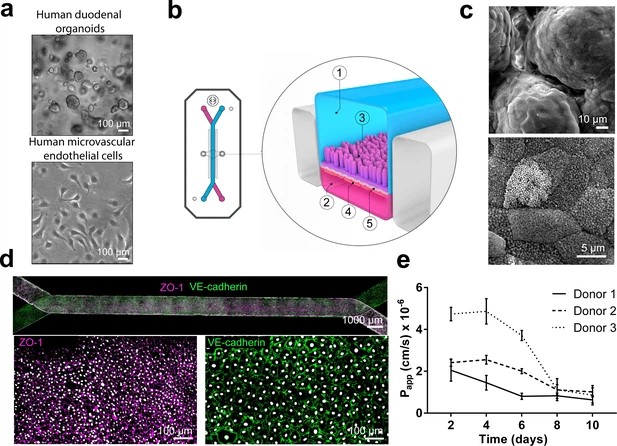 Fig. 1. Duodenum Intestine-Chip: a microengineered model of the human duodenum (Kasendra M, Luc R, et al., 2020).
Fig. 1. Duodenum Intestine-Chip: a microengineered model of the human duodenum (Kasendra M, Luc R, et al., 2020).
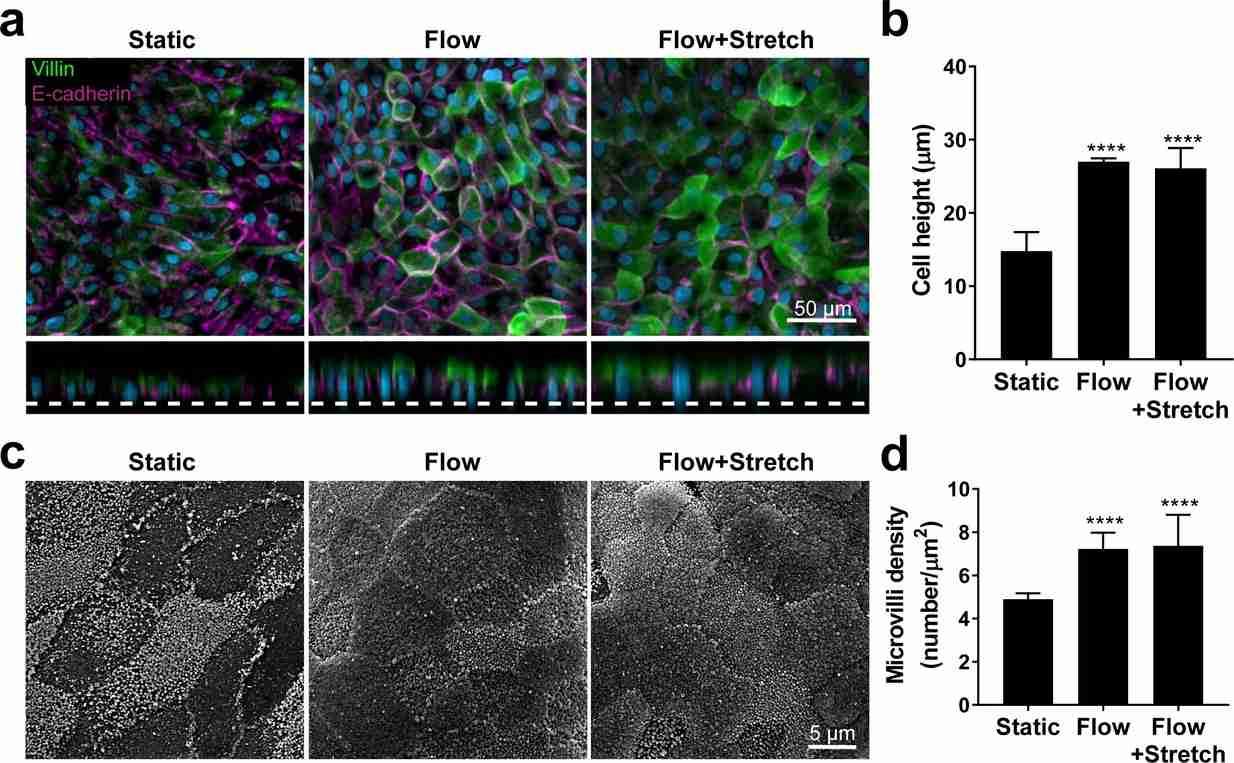 Fig. 2. Flow-induced increase in primary human small intestinal epithelial cells height and microvilli formation (Kasendra M, Luc R, et al., 2020).
Fig. 2. Flow-induced increase in primary human small intestinal epithelial cells height and microvilli formation (Kasendra M, Luc R, et al., 2020).
Neonatal-intestine-on-a-chip Models Human Small Intestinal Architecture
Necrotizing enterocolitis (NEC) is a severe intestinal disease in premature infants, characterized by excessive inflammation, bacterial imbalance, and epithelial damage leading to a disrupted gut barrier. Lanik et al. developed a Neonatal-Intestine-on-a-Chip to model NEC, which utilizes a combination of premature infant small intestinal enteroids cocultured with human small intestinal microvascular endothelial cells and patient-derived microbiota to recreate critical features of premature gut pathophysiology, including microbial dysbiosis.
To establish baseline cellular growth, they tracked the development of intestinal stem cells from enteroids (day 0) to neonatal epithelium (day 8) using brightfield microscopy (Fig. 3A). On day 0, enteroids are distinct organoids. By day 1, they were dissociated and seeded into a Matrigel-coated microfluidic device, forming a confluent monolayer by day 3. Furrows appeared by day 6, progressing to a 3D architecture with villus-like structures by day 8 (Fig. 3A). To assess architectural similarity to the human small intestine, they immunostained cells for villi markers: villin (green) and FABP1 (white), with counterstaining by phalloidin (red) and Hoechst 33342 (blue; Fig. 3B). The resulting structure resembles human intestinal villi with microvilli-lined enterocytes. They examined polarity within the Neonatal-Intestine-on-a-Chip using ZO-1 (red) for apical junctions and β-catenin (green) for basolateral junctions (Fig. 3C). Confocal microscopy showed ZO-1 near the lumen and β-catenin near the semipermeable membrane, indicating key neonatal intestinal features such as 3D architecture, cell adhesion, and polarity.
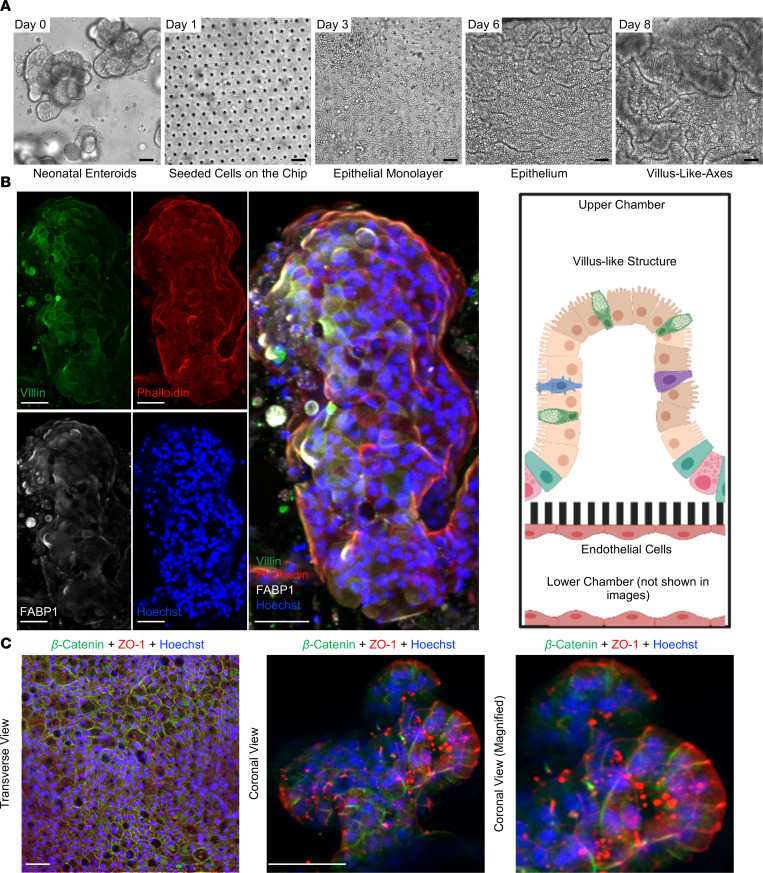 Fig. 3. Development of the Neonatal-Intestine-on-a-Chip microfluidic model (Lanik W E, Luke C J, et al., 2023).
Fig. 3. Development of the Neonatal-Intestine-on-a-Chip microfluidic model (Lanik W E, Luke C J, et al., 2023).
Ask a Question
Write your own review