ONLINE INQUIRY

Human Coronary Artery Endothelial Cells
Cat.No.: CSC-C8595W
Species: Human
Source: Artery
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Coronary artery disease is the most common type of cardiovascular disease (CVD) that can lead to myocardial infarction, defined by the death of myocardial cells due to prolonged ischemia. The extent of ischemia-induced myocardial damage depends greatly on the duration of ischemia and efficient tissue repair. Therefore, a timely and robust repair response is necessary to maintain normal organ function. Specifically, successful reperfusion paired with neovascularization after myocardial infarction are necessary not only to repair ischemic myocardium, but also to prevent heart failure by promoting left ventricular remodeling.
The coronary endothelium plays an important role in the repair response following an ischemic event. Under physiological conditions, endothelial cells (ECs) are quiescent and thus help maintain blood flow and barrier function while counteracting thrombosis and vascular inflammation. However, following a myocardial event, ECs become activated, increasing vascular permeability and angiogenesis. After the initial inflammatory response, a repair phase ensues, resulting in attenuation of inflammation, fibroblast proliferation, and neovascularization via angiogenesis. Angiogenesis is a process that is tightly regulated by multiple cues such as hypoxia, vascular endothelial growth factor (VEGFA), nitric oxide (NO), or other local mediators. When exposed to angiogenic signals, quiescent ECs become activated, loosening their tight junctions, and thus allowing extravasation of plasma proteins forming a temporary matrix. This matrix allows specialized ECs, termed tip cells, to migrate to initiate the formation of a new sprout. In addition, adjacent ECs, stalk cells, proliferate to further elongate the sprout. The individual elongated sprouts will then coalesce to form the lumen and new vascular networks. Thus, cell migration, proliferation and increased vascular permeability contribute concertedly to a successful angiogenic response.
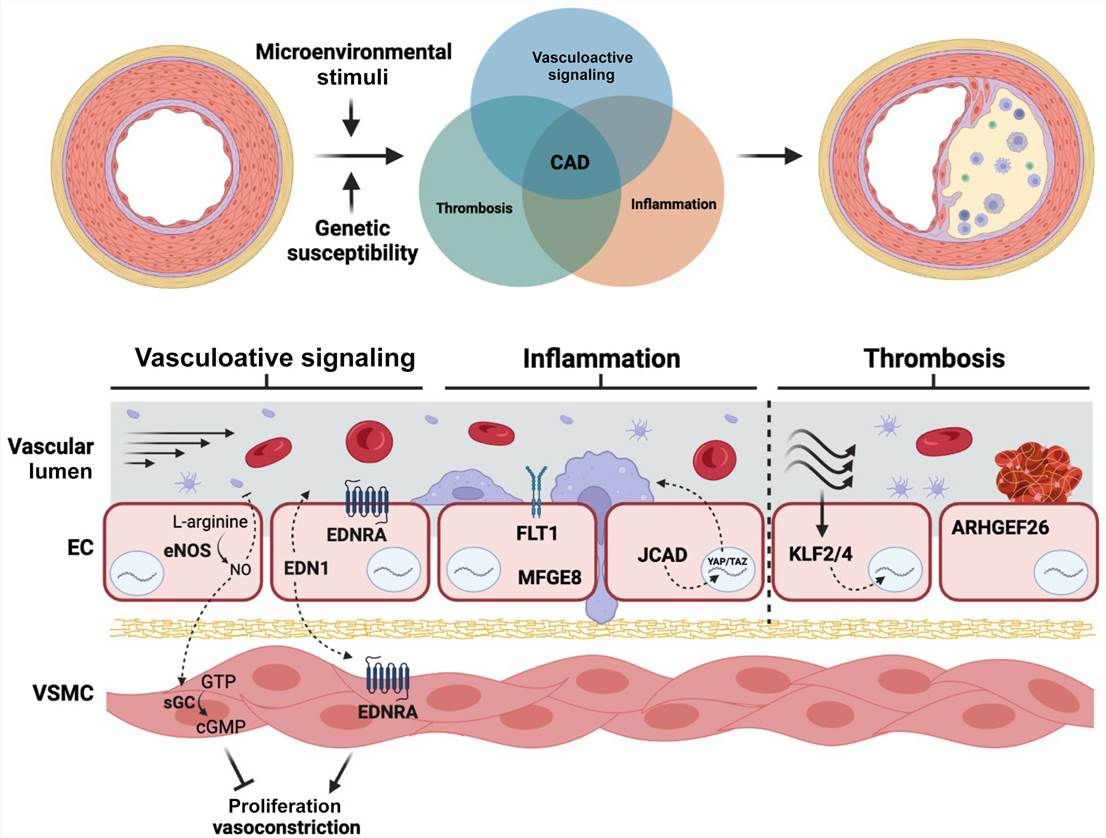 Fig. 1. Genetic risk variants affect different endothelial cell functions that contribute to atherosclerotic risk (Pepin, Mark E. and Rajat M. Gupta. 2024).
Fig. 1. Genetic risk variants affect different endothelial cell functions that contribute to atherosclerotic risk (Pepin, Mark E. and Rajat M. Gupta. 2024).
Over the past few decades, the majority of endothelial proinflammatory/coagulation studies have been performed on human umbilical vein endothelial cells (HUVECs), derived from vascular beds not present in the adult, making these cells inappropriate as models of endothelial inflammation and coagulation. Human coronary artery endothelial cells (HCAECs) have previously been shown to be strikingly greater responsiveness than HUVECs.
Effects of Different Doses of HCY on Cell Apoptosis of HCAECs
Homocysteine (HCY) is capable of inducing the apoptosis in endothelial cells, and it could be an essential mechanism for development of cardiovascular diseases. After treatment with different doses of HCY for 24 h, 48 h and 72 h, the activation of HCAECs was detected using an MTT assay. The results showed that the activation of HCAECs receiving HCY concentrations of 0.1, 0.25, 0.5 or 1.0 mmol/L was decreased, and in 24 h, a significant difference was found between the proliferation rate of HCAECs treated with HCY concentrations of 0.5 and 1.0 mmol/L and that of the control group (HCY concentration 0 mmol/L) (both P < 0.01). Compared with the control group (0 mmol/L), the cell proliferation rates after HCY treatment (0.25, 0.5 and 1.0 mmol/L) were significantly different at 48 h (all P < 0.05) (Fig. 1).
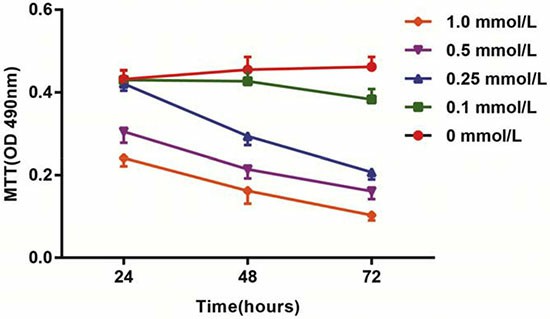 Fig. 1. Effects of different doses (0, 0.1, 0.25, 0.5 and 1.0 mmol/L) of HCY on cell activity of HCAECs (Song, Chun-Li, et al., 2016).
Fig. 1. Effects of different doses (0, 0.1, 0.25, 0.5 and 1.0 mmol/L) of HCY on cell activity of HCAECs (Song, Chun-Li, et al., 2016).
Hoechst 33258 is a nucleic acid-specific dye. The nuclei in normal cells presented uniformly hypochromatic blue color, and the nuclei in apoptotic cells presented a series of typical apoptosis characteristics, such as pyknosis, chromatin enrichment and apoptotic bodies (Fig. 2A). After treatment with different doses of HCY (0.1, 0.25, 0.5 and 1.0 mmol/L) for 24 h, the apoptosis rates (12.8 ± 2.5%, 17.4 ± 2.8%, 20.5 ± 3.6%, 27.8 ± 4.7%, respectively) were significantly increased compared with the apoptosis rate (6.9 ± 2.1%) in the control group (0 mmol/L) (all P < 0.05) (Fig. 2B).
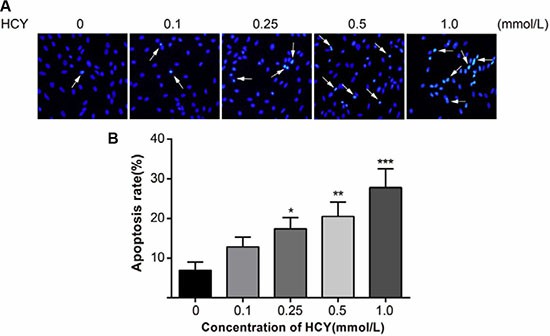 Fig. 2. Effect of different doses (0, 0.1, 0.25, 0.5 and 1.0 mmol/L) of HCY on cell apoptosis of HCAECs (Song, Chun-Li, et al., 2016).
Fig. 2. Effect of different doses (0, 0.1, 0.25, 0.5 and 1.0 mmol/L) of HCY on cell apoptosis of HCAECs (Song, Chun-Li, et al., 2016).
Regulation of Apoptosis in Cultured HCAECs by Estradiol
To investigate the role of estradiol in cultured HCAECs, we first used the TUNEL assay to assess DNA breaks indicative of early-stage apoptosis under basal and 17β-estradiol (E2)-treated conditions. TUNEL assay showed that treatment of HCAECs with E2 (10-10 and 10-8 M) induced a concentration- and time-dependent increase in the percentage of TUNEL-positive HCAEC percentage after 24- and 72-h treatments (P < 0.05, Fig. 3). At the end of 72-h treatment, E2 at 10-8 M enhanced the proportion of TUNEL-positive cells by 2.8 ± 0.2-fold (P < 0.05, mean ± SEM).
We then performed DAPI staining to analyze late-stage morphological indicators of apoptosis such as nuclear segmentation and chromatin condensation. Similar to the results of TUNEL labeling, E2-treated (10-10 to 10-8 M) HCAECs had significantly more cell shrinkage, nuclear segmentation, and chromatin condensation, compared with control cells at 24 and 72 h (Fig. 3, P < 0.05). The proportion of apoptotic cells was increased by 2.2 ± 0.3-fold (mean ± SEM) after a 72-h treatment with E2 at 10-8 M (P < 0.05).
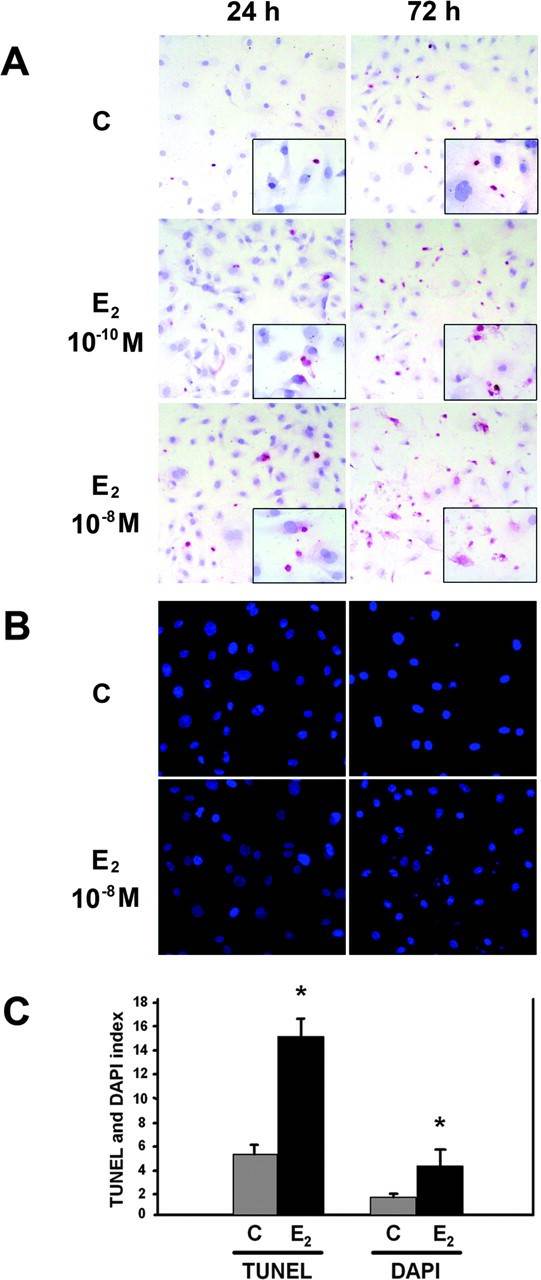 Fig. 3. The effect of E2 on HCAEC apoptosis assessed by TUNEL assay and DAPI fluorescence (Seli, Emre, et al., 2006).
Fig. 3. The effect of E2 on HCAEC apoptosis assessed by TUNEL assay and DAPI fluorescence (Seli, Emre, et al., 2006).
Regulation of Fas Protein and mRNA Expression by Estradiol in Cultured HCAECs
To evaluate the regulation of Fas protein expression by E2, HCAECs were incubated with vehicle (control) or various concentrations of E2 (10-12 to 10-8 M) for 12–48 h, and Fas protein levels were analyzed by Western blot analysis. E2 treatments induced a significant concentration-dependent increase in Fas protein expression, compared with controls at all time points investigated (P < 0.05, Fig. 4).
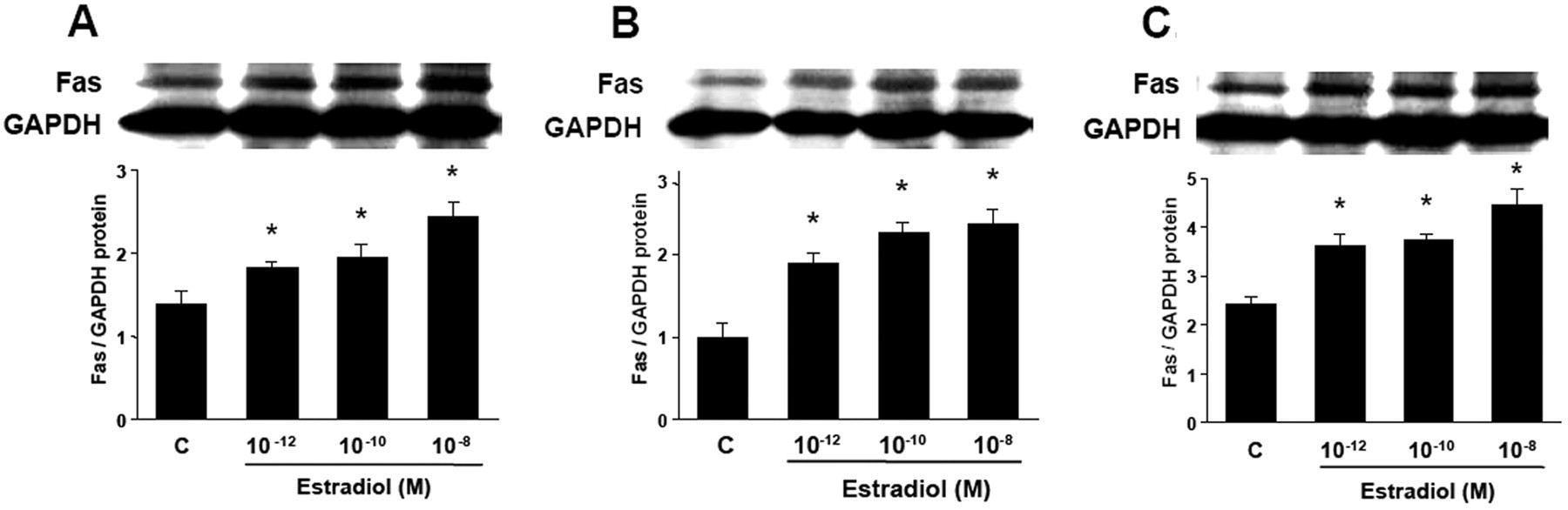 Fig. 4. Western blot analysis for Fas in cultured HCAECs (Seli, Emre, et al., 2006). A 12 h, B 24 h, C 48 h
Fig. 4. Western blot analysis for Fas in cultured HCAECs (Seli, Emre, et al., 2006). A 12 h, B 24 h, C 48 h
To assess whether the up-regulatory effect of E2 on Fas protein expression was secondary to an increase in Fas mRNA transcription, Fas mRNA levels were measured by RT-PCR analysis. HCAECs were incubated with vehicle (control) or various concentrations of E2 (10-12 to 10-8 M) for 8 h. Semiquantitative RT-PCR results corresponded to Western blot analysis findings. Fas mRNA expression was increased by 65 ± 4 and 84 ± 8% with E2 treatment at 10-10 and 10-8 M concentrations, respectively (P < 0.05, Fig. 5).
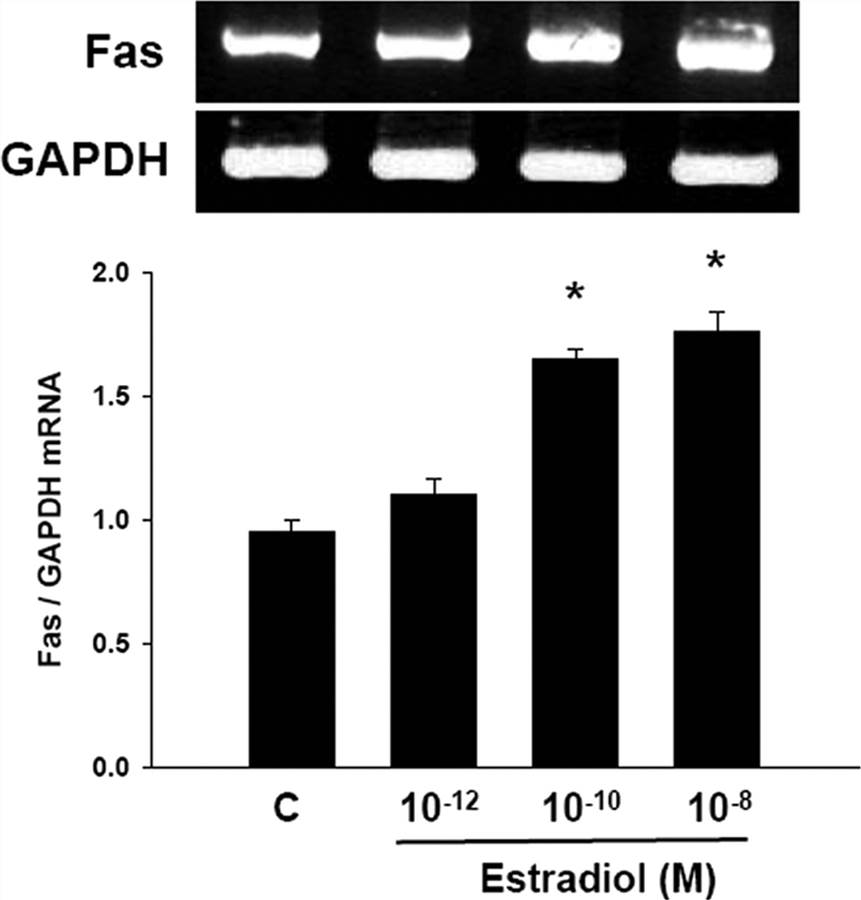 Fig. 5. Semiquantitative RT-PCR for Fas in cultured HCAECs (Seli, Emre, et al., 2006).
Fig. 5. Semiquantitative RT-PCR for Fas in cultured HCAECs (Seli, Emre, et al., 2006).
Additional donor characteristics including hypertension (cat# CSC-C4986J) are available by contacting our Scientific Support Team.
Angiogenesis, arteriolosclerosis, hypertension, oncology, drug screening, inflammation, vasodilator function.
Check all containers for leakage or breakage. Directly and immediately transfer the cells from dry ice to liquid nitrogen and keep the cells in liquid nitrogen until they are needed for experiments.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Reliable products
I am confident in the integrity of my research data thanks to the reliable cells obtained from Creative Bioarray, and I plan to continue using their products in future projects.
20 May 2023
Ease of use
After sales services
Value for money
Write your own review

