ONLINE INQUIRY

Human Aortic Adventitia Fibroblasts (HAAF)
Cat.No.: CSC-7839W
Species: Human
Source: Aorta
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Despite significant advances in treatment, arterial diseases remain major causes of morbidity and mortality worldwide. Recently, adventitial fibroblasts (AFs) have been implicated as major contributors to the maladaptive cascades that occur in arterial disease, vessel remodeling, and restenosis following vascular interventions.
In fact, AFs are the first vascular wall cells to exhibit signs of activation following physiologic stress. Activated AFs can transdifferentiate into myofibroblasts and secrete a variety of signaling factors, including cytokines, growth factors, and proteases, that alter the behaviors of other resident adventitial cells, and ultimately disturb vascular pathobiology. AFs alter the structure and function of the vessel wall in multiple ways to alter tissue stiffness, especially by balancing the production of extracellular matrix (ECM) proteins and ECM-degrading proteases, such as matrix metalloproteinases (MMPs). Conversely, the matrix stiffness can significantly alter the expression of AF gene and the generation of cytokines. Thus, AFs both alter substrate stiffness and respond to it in the context of adventitial function and vascular disease progression.
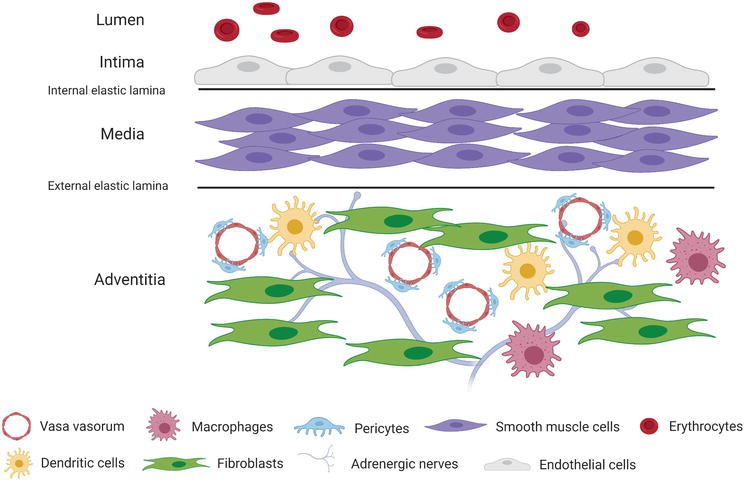 Fig. 1. Heterogeneous cellular composition of the vascular adventitia (Tadeja, K., and S. Š. Snezna. 2021).
Fig. 1. Heterogeneous cellular composition of the vascular adventitia (Tadeja, K., and S. Š. Snezna. 2021).
Complicating the in vitro study of fibroblasts is the fact that there are currently no fibroblast-specific markers for identification. In addition, fibroblasts tend to transform when removed from the tunica adventitia, which is considered to be mainly in response to mechanical stress in in vitro cell culture conditions. Thus, improved methods to identify and maintain fibroblasts in culture are crucial to increase the translational applicability of cellular studies involving adventitial fibroblasts.
To study the role of matrix stiffness on human aortic adventitial fibroblast (HAoAF) fate, 3D cell-laden hydrogels were formed under physiological conditions via a Michael-type addition reaction using thiol end-functionalized PEG macromers (PEG-SH4) and bis-maleimide end-functionalized, MMP-sensitive crosslinker peptides (PQ-MI2) to obtain highly elastic hydrogels that accommodated cell-mediated remodeling of the matrix via protease degradation (Fig. 1). As collagen is the predominant structural and cell-adhesive protein found in arterial adventitia and a key mediator of HAoAF activities, 1 mM mono-maleimide pendent RGD peptide (RGD-MI), an adhesive motif found within collagen, was incorporated into the polymer matrix to facilitate HAoAF interactions with the hydrogel network.
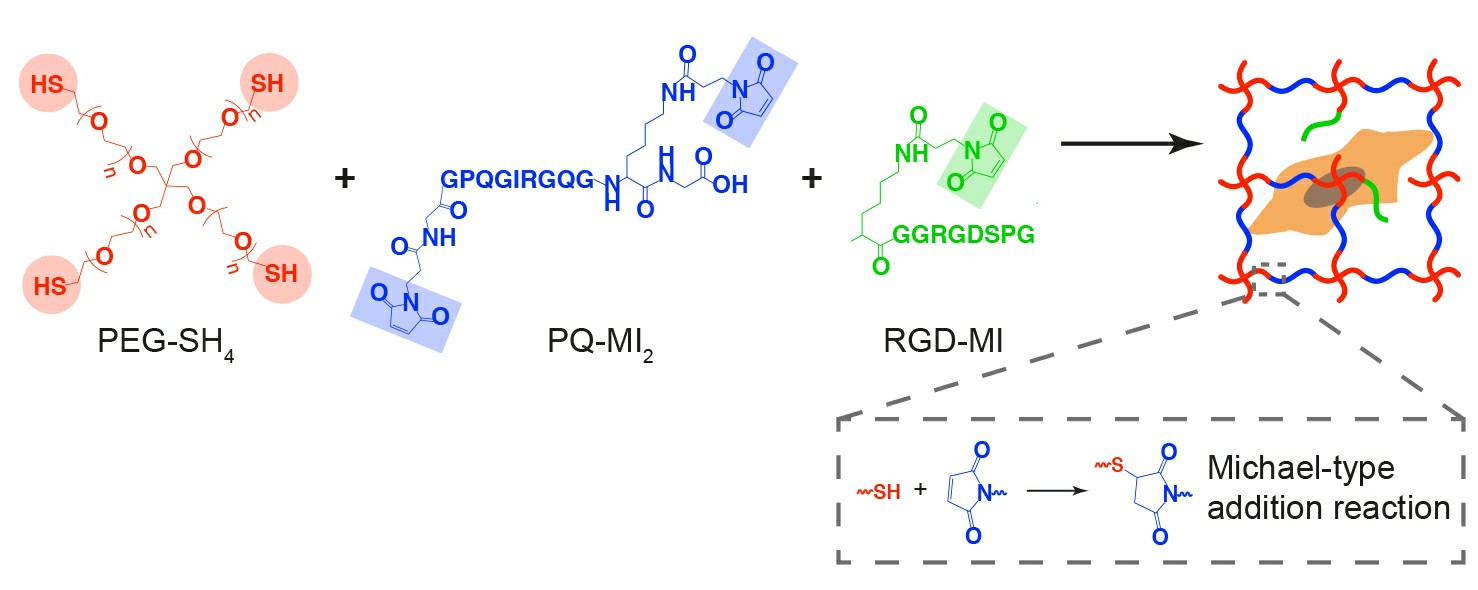 Fig. 1. Reaction scheme for enzymatically degradable hydrogel formation (Scott, Rebecca A., et al., 2020).
Fig. 1. Reaction scheme for enzymatically degradable hydrogel formation (Scott, Rebecca A., et al., 2020).
We next sought to determine whether differences in initial matrix stiffness provoked HAoAF activation just after culture initiation by assessing cell proliferation and secretion of key cytokines, including the angiogenic growth factor vascular endothelial growth factor (VEGF-A), and the inflammatory cytokines interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1). Elevated proliferation was observed in soft 5wt% hydrogels in the initial 7 days following encapsulation and proliferation decreased as hydrogel stiffness increased (Fig. 2A), similar to previous reports with fibroblasts in 3D. We further observed that elevated HAoAF proliferation in 5wt% hydrogels corresponded with enhanced production of the angiogenic growth factor, VEGF-A (detected via enzyme-linked immunosorbent assay (ELISA)), compared to 7.5wt% and 10wt% hydrogels (Fig. 3), which is consistent with in vivo reports where increased adventitial cell proliferation corresponded to increased VEGF-A production.
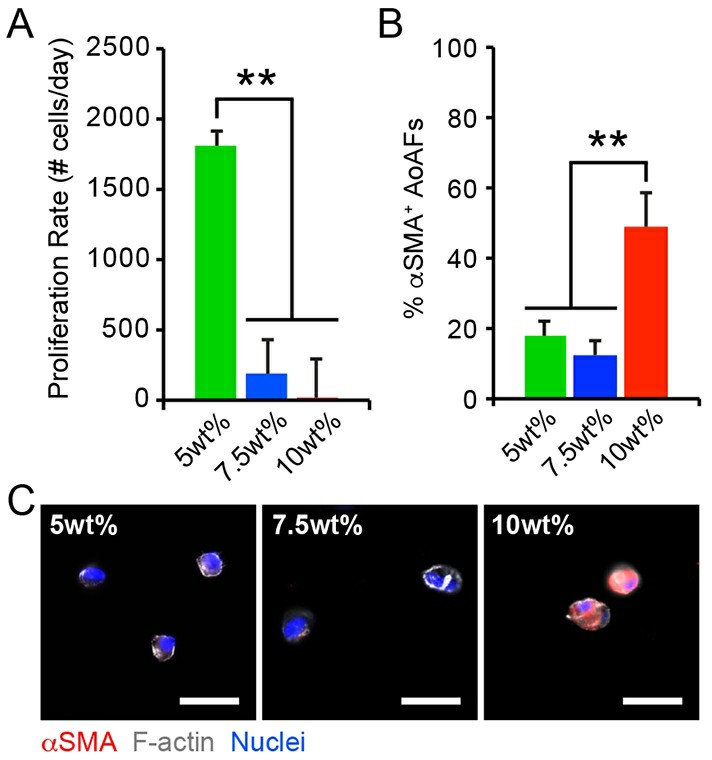 Fig. 2. (A) The proliferation rate of HAoAF encapsulated in 5wt%, 7.5wt%, and 10wt% hydrogels significantly decreased with increasing weight percent over 7 days. (B-C) The percentage of αSMA+ HAoAF was significantly increased in 10wt% hydrogels compared to softer 5wt% and 7.5wt% substrates after 1 day, as confirmed by immunocytochemistry (Scott, Rebecca A., et al., 2020). Blue: Hoechst 33258 (nuclei), Grey: F-actin, Red: αSMA.
Fig. 2. (A) The proliferation rate of HAoAF encapsulated in 5wt%, 7.5wt%, and 10wt% hydrogels significantly decreased with increasing weight percent over 7 days. (B-C) The percentage of αSMA+ HAoAF was significantly increased in 10wt% hydrogels compared to softer 5wt% and 7.5wt% substrates after 1 day, as confirmed by immunocytochemistry (Scott, Rebecca A., et al., 2020). Blue: Hoechst 33258 (nuclei), Grey: F-actin, Red: αSMA.
Under physiologic and pathophysiologic conditions, alterations to the adventitia are often accompanied by the appearance of proliferative cells expressing α-smooth muscle actin (αSMA), a marker of fibroblast to myofibroblast transition, and increased matrix deposition. Though HAoAF in the 5wt% hydrogels exhibited enhanced proliferation, immunostaining revealed that few cells (<20%) expressed αSMA in 5wt% and 7.5wt% hydrogels 24 hrs post-encapsulation (Fig. 2B-C). On the other hand, ~50% of HAoAF in the stiffer 10wt% hydrogels expressed diffuse αSMA staining after 24 hrs. While our results differ from previous observations in 3D hydrogels, where fibroblast activation decreased with increased stiffness, they are consistent with in vivo observations of pathologic tissue stiffening driving myofibroblast trans-differentiation. Further, αSMA expression in 10wt% hydrogels was accompanied by the secretion of the inflammatory proteins, IL-6 and MCP-1 (measured via ELISA; Fig. 3B-C). Interestingly, significantly increased IL-6 and MCP-1 production by HAoAF encapsulated in 5wt% hydrogels, compared to 7.5wt% hydrogels, suggests that inflammatory cytokine production by activated HAoAF may be regulated through pathways distinct from those driving myofibroblast trans-differentiation.
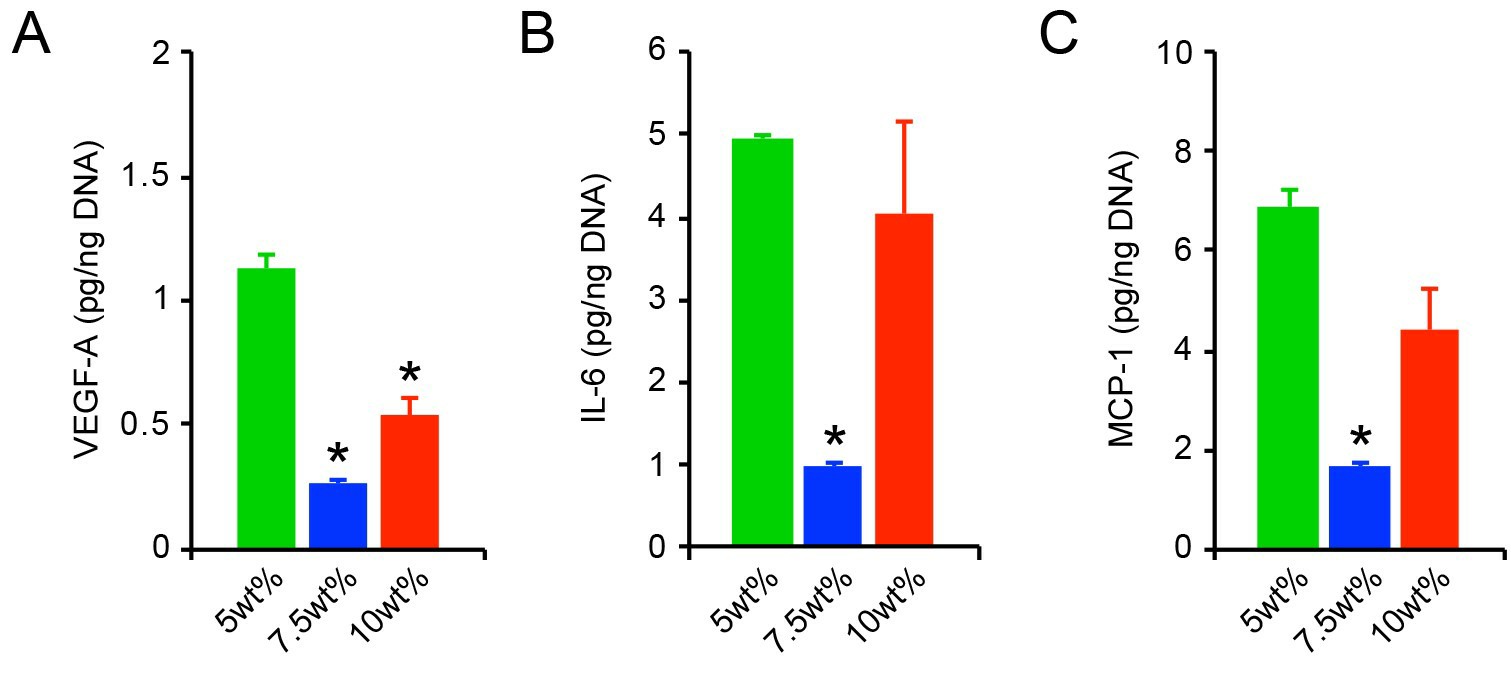 Fig. 3. HAoAF secretion of (A) VEGF-A, (B) IL-6, and (C) MCP-1, following culture for 1 day in 5wt%, 7.5wt%, and 10wt% hydrogels (Scott, Rebecca A., et al., 2020).
Fig. 3. HAoAF secretion of (A) VEGF-A, (B) IL-6, and (C) MCP-1, following culture for 1 day in 5wt%, 7.5wt%, and 10wt% hydrogels (Scott, Rebecca A., et al., 2020).
Within native tissues and tissue mimetic substrates, cells actively respond to their surroundings by synthesizing and secreting ECM proteins. Accordingly, we assessed the distribution of collagen I and collagen III, which are the main collagen constituents in the adventitia. Following encapsulation, collagen I and collagen III immunofluorescent staining was primarily localized to the intracellular or pericellular regions in all cultures (Fig. 4D-E). Quantification of collagen levels by corrected total cell fluorescence (CTCF) revealed that collagen I expression was increased among HAoAF in 5wt% hydrogels after 1 day, but then decreased as hydrogel stiffness increased (Fig. 4F). Collagen III expression was also increased in 5wt% hydrogels, compared to cells in 7.5% and 10wt% hydrogels (Fig. 4G). Overall, these results are in agreement with previous findings where substrate stiffness regulates ECM protein production, and support the notion that initial hydrogel conditions strongly impact the composition of the secreted ECM.
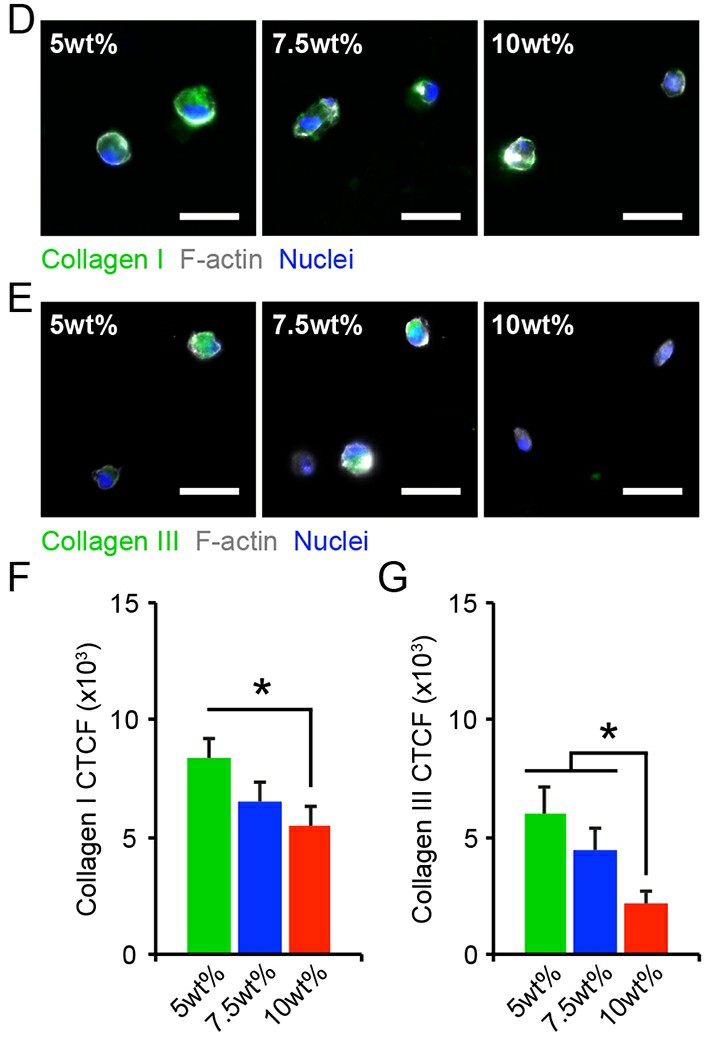 Fig. 4. (D-E) HAoAF produced collagen I or collagen III, detected via immunocytochemistry, following encapsulation in 5wt%, 7.5wt%, and 10wt% hydrogels for 1 day. Blue: Hoechst 33258 (nuclei), Grey: F-actin, Green: collagen I or collagen III. (F-G) Expression of collagen I and collagen III expression, measured by corrected total cell fluorescence, decreased with increasing weight percent (Scott, Rebecca A., et al., 2020).
Fig. 4. (D-E) HAoAF produced collagen I or collagen III, detected via immunocytochemistry, following encapsulation in 5wt%, 7.5wt%, and 10wt% hydrogels for 1 day. Blue: Hoechst 33258 (nuclei), Grey: F-actin, Green: collagen I or collagen III. (F-G) Expression of collagen I and collagen III expression, measured by corrected total cell fluorescence, decreased with increasing weight percent (Scott, Rebecca A., et al., 2020).
We have a variety of fibroblast media to choose from, including base medium (cat# CM-1037X), low serum medium (cat# CM-1036X) and serum-free medium (cat# CM-1035X), please contact our staff for more information.
Our Human Aortic Adventitia Fibroblasts (HAAF) are isolated from human aortic artery, cryopreserved at first passage of primary culture and delivered frozen.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
It gives outstanding results
The cell did as expected in our study and it gave good results.
17 Apr 2022
Ease of use
After sales services
Value for money
Write your own review

