Human Aortic Endothelial Cells
Cat.No.: CSC-C4862L
Species: Human
Source: Aorta
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Human aortic endothelial cells (HAECs) derive from the endothelial tissue of the human aorta, the largest artery in the body that carries oxygen-rich blood from the heart to the rest of the body. HAECs are adherent-growing epithelioid or polygonal cells that are spindle-like or densely packed under the microscope and positive for Factor VIII staining. HAECs regulate vascular contraction and growth. They release a wide range of bioactive substances, including antithrombotic and prothrombotic factors like tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor-1 (PAI-1). Also, HAECs modulate and regulate inflammation by activating cell adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1). Furthermore, HAECs are also expressed with signaling biomarkers, including Tie2, eNOS, Axl and Etk/Bmx that are important for angiogenesis, cell survival, migration, proliferation and signaling.
Therefore, HAECs are widely used in cardiovascular function and disease studies, particularly diabetes complications, immune response, rejection of transplants, endothelial dysfunction, and for 3D endothelialized engineered tissues, new material surfaces and drug technologies. Those cells are also critical for angiogenesis and other important endothelial cell signaling networks.
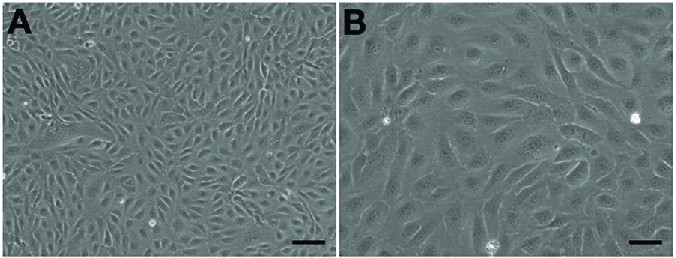 Fig. 1. Morphology of human aortic endothelial cells under microscope in (A) 40×, Scale bar = 100 µm; (B) 100×, Scale bar = 50 µm, showed classical round borders and cobblestone appearance (Hao Q, Wang H, et al., 2019).
Fig. 1. Morphology of human aortic endothelial cells under microscope in (A) 40×, Scale bar = 100 µm; (B) 100×, Scale bar = 50 µm, showed classical round borders and cobblestone appearance (Hao Q, Wang H, et al., 2019).
Glucose Treatment Causes Significant Transcriptional Changes in HAECs
Diabetes type 2 is a rising worldwide epidemic, with elevated blood sugar a primary risk factor for issues like vascular disease. Dysfunction of endothelial cells is crucial to these glucose-driven disorders. Bayaraa's team used deep time-series RNA-seq analysis to analyze human aortic endothelial cells in 24 hours exposed to normal and high glucose, looking for gene clusters impacted by glucose and the precise signaling cascades involved in initial transcriptional responses.
In order to examine the transcriptome change of human aortic endothelial cells (HAECs) in response to either normal glucose (NG, 5.5 mM) or high glucose (HG, 25 mM), they performed a time-course RNA-seq assay (Fig. 1). HAECs were exposed overnight to reduced serum and treated with NG or HG for 0.5, 1, 4, 8, and 24 hours. Each time point had four replicates, except 0.5 h HG with three. PCA showed distinct clusters related to treatment duration (Fig. 2A), indicating significant transcriptional changes as early as 0.5 hours, peaking at 4 hours (Fig. 2A). Differential gene expression analysis compared baseline (NG 0 h) to each time point. Under NG, DEGs peaked at 4 hours (1875 genes) and decreased by 8 hours (1203) and 24 hours (713). Initially, NG showed many upregulated genes (88% at 0.5 h), which balanced later (42–54%, Fig. 2B). Under HG, a similar pattern occurred with more DEGs, peaking at 4 hours with 2250 genes (Fig. 2B). For both NG and HG conditions, pairwise comparisons between time points (0–0.5, 0.5–1, 1–4, 4–8, and 8–24 h) were conducted to examine changes in the number of DEGs relative to prior intervals (Fig. 2C). A similar expression pattern was observed in both conditions, akin to the analysis against the baseline.
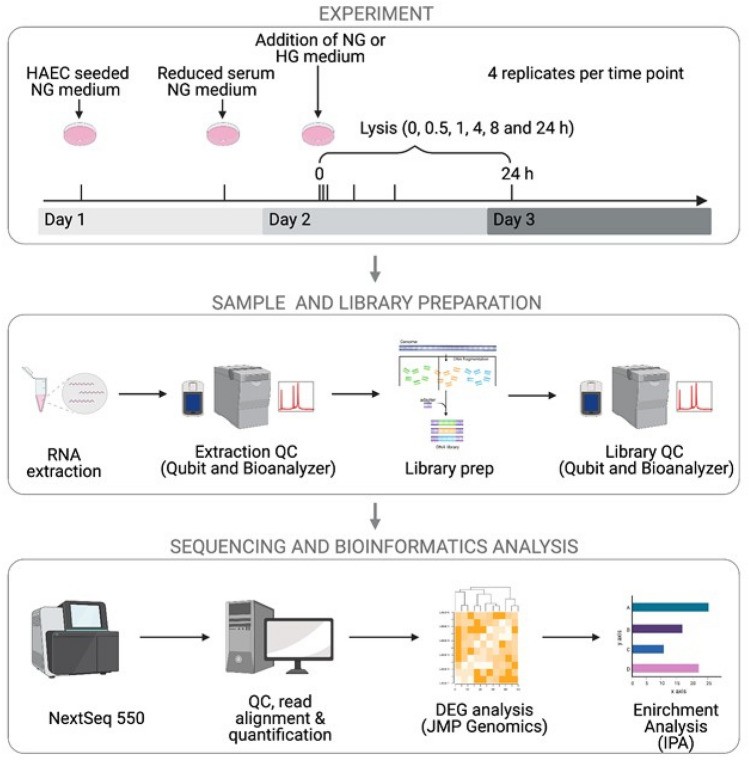 Fig. 1. Schematic of time-series RNA-seq experimental design (Bayaraa O, Inman C, et al., 2022).
Fig. 1. Schematic of time-series RNA-seq experimental design (Bayaraa O, Inman C, et al., 2022).
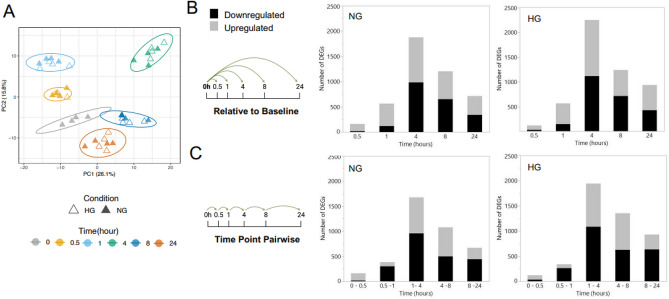 Fig. 2. The temporal effect of glucose treatment on the HAEC transcriptome (Bayaraa O, Inman C, et al., 2022).
Fig. 2. The temporal effect of glucose treatment on the HAEC transcriptome (Bayaraa O, Inman C, et al., 2022).
Curcuminoids Inhibit Tubulogenesis in Stimulated HAECs
Angiogenesis, a key factor in tumor progression, can be inhibited by natural compounds such as curcumin, which affects endothelial cell functions. However, the effects of other curcuminoids like DMC and BisDMC remain underexplored. Giménez-Bastida et al. used human aortic endothelial cells (HAECs) to investigate the antiangiogenic activities of curcumin and its derivatives at physiological levels.
They examined how Curc, DMC, BisDMC, and their combination (Mix) at 5 μM affect the tubulogenic ability, specifically ring formation, of HAECs at various time points (Fig. 3). Without pro-angiogenic factors, there were no differences after 4 hours of pretreatment with curcuminoids between treated and untreated cells (Fig. 3B). However, when stimulated, cells formed more ring-like structures over time. All compounds significantly inhibited ring formation at different time points (Fig. 3A and B). They further tested lower concentrations (1 and 0.1 μM) at 21 hours, finding Curc ineffective below 5 μM (Fig. 4). In contrast, DMC and BisDMC consistently inhibited tubulogenesis across concentrations, while Mix showed significant effects at 5 and 1 μM (Fig. 4D).
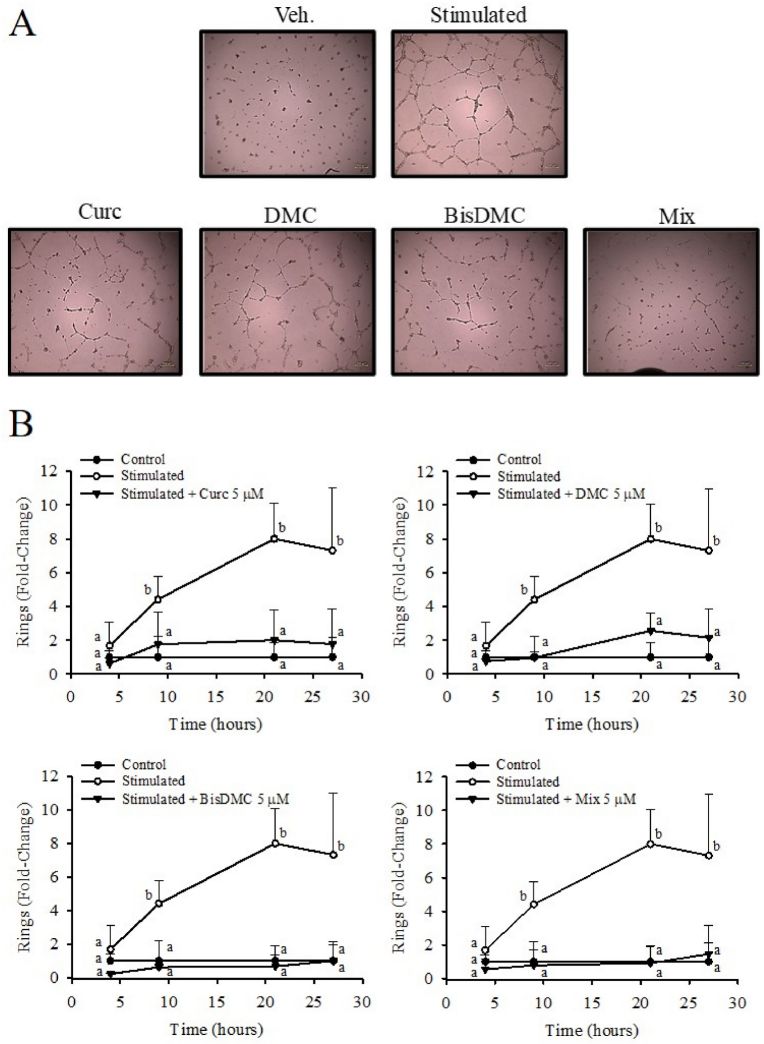 Fig. 3. Time course effect of Curc, DMC, BisDMC, and the Mix on HAECs tubulogenesis (iménez-Bastida J A, Ávila-Gálvez M A, et al., 2022).
Fig. 3. Time course effect of Curc, DMC, BisDMC, and the Mix on HAECs tubulogenesis (iménez-Bastida J A, Ávila-Gálvez M A, et al., 2022).
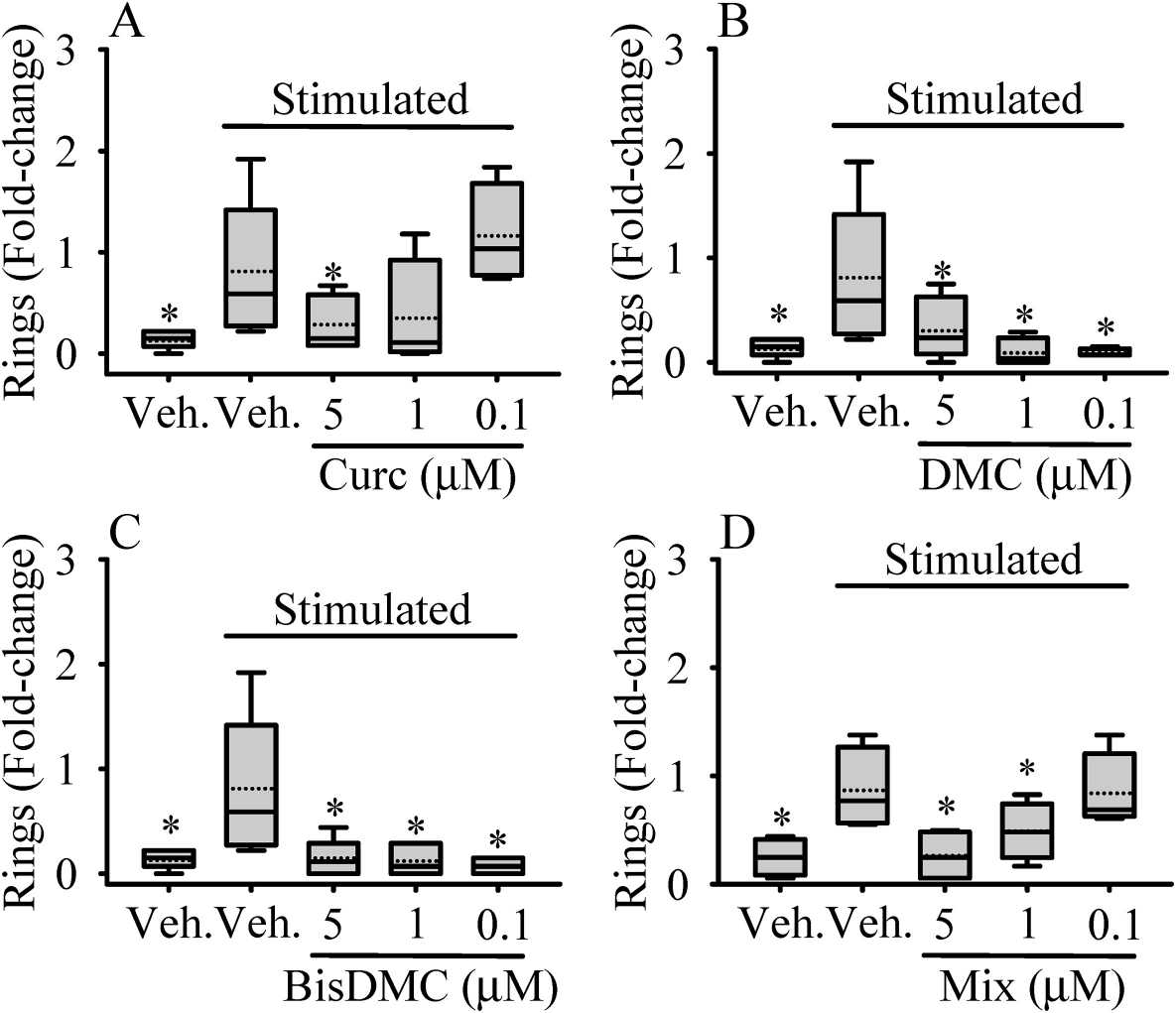 Fig. 4. Dose-dependent effect of Curc (A), DMC (B), BisDMC (C) and the Mix (D) on HAECs tubulogenesis (iménez-Bastida J A, Ávila-Gálvez M A, et al., 2022).
Fig. 4. Dose-dependent effect of Curc (A), DMC (B), BisDMC (C) and the Mix (D) on HAECs tubulogenesis (iménez-Bastida J A, Ávila-Gálvez M A, et al., 2022).
Ask a Question
Write your own review

