
C57BL/6 Mouse Bone Marrow Dendritic Cells
Cat.No.: CSC-C4971J
Species: Mouse
Source: Bone Marrow
Cell Type: Dendritic Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
C57BL/6 mouse bone marrow dendritic cells are harvested from the bone marrow of this erect mouse breed, known for its fertility, anti-disease resistance and versatility. Their morphology is typical of dendritic cells, with their own distinct dendritic protrusions, giving rise to more cell surface area and greater interactions with other immune cells.
Once they mature bone marrow, these cells move outward to peripheral immune organs like the spleen and lymph nodes to carry out their work. They are highly immunologically active, effectively identifying foreign antigens. Through their surface pattern recognition receptors, they capture antigens, process them, and present the antigen peptides bound to major histocompatibility complex (MHC) molecules on their surface to activate T-cell-mediated immune responses. Moreover, they are able to modulate their immune systems in response to microenvironmental cues, for example, by producing or inhibiting various cytokines during inflammation. In research use, C57BL/6 mouse are common experimental animals, and bone marrow dendritic cells of the mouse provide insights into immune cell-cell communication, mechanisms of activating and deactivating immune tolerance, and so on. These cells can be used in cancer immunotherapy studies to determine how new immunotherapies affect dendritic cell activity, and to identify ways to maximize cancer immunotherapy.
Maturation Marker Analysis and Cytokines Released by BMDC-dLN
In recent decades, decellularized extracellular matrix (ECM) scaffolds have shown potential for use in tissue regeneration. As cancer immunotherapy emerging as a more targeted treatment against cancer, dendritic cell (DC)-based therapies hold promise due to their unique role in immune modulation. However, several drawbacks restrict the clinical efficacy of DC therapy, the in vitro culture cannot generate sufficiently powerful DCs is among these. Lin's team developed a natural decellularized lymph node (dLN) scaffold capable of supporting dendritic cell proliferation and maturation in vitro, and confirmed its ability to induce a specific antitumor immune response in vivo using bone marrow dendritic cells.
dLN scaffolds were obtained by decellularization of rat lymph nodes with FA, AA, and CA. Bone marrow dendritic cells (BMDCs) were subsequently injected into the scaffold and cultured for 3 days. Hoechst and anti-CD11c immunofluorescence staining revealed that the BMDC grew preferentially inside the dLN scaffolds (Fig. 1A). Co-stimulatory molecules CD80 and CD86 are essential markers of DC maturation, signifying their capacity to trigger antigen-specific immunity. CD80 expression on BMDC-dLN increased with OVA antigen stimulation (Fig. 1B), and CD86 expression similarly rose with higher OVA doses (Fig. 1C). Another important marker for activated DCs is the MHC molecule, which helps T lymphocytes recognize antigens. MHC-II expression in BMDC was notably high in cultures containing OVA antigen and CPG-ODN (Fig. 1D). This indicates significant up-regulation of maturation markers in BMDC, enhancing their ability to induce immune responses (Fig. 1E). Additionally, the cytokine release profile of BMDC-dLN was crucial. When stimulated with various concentrations of OVA antigen and CPG-ODN, BMDC-dLN produced more pro-inflammatory cytokines IL-1β and IL-6 than the control (Fig. 2A and B). IL-12, vital for naive T helper lymphocyte activation, was also significantly released by BMDC-dLN treated with 100 µg/mL OVA (Fig. 2C). These findings indicate that BMDCs thrived within the dLN, maturing effectively and inducing a robust immune response upon antigen stimulation.
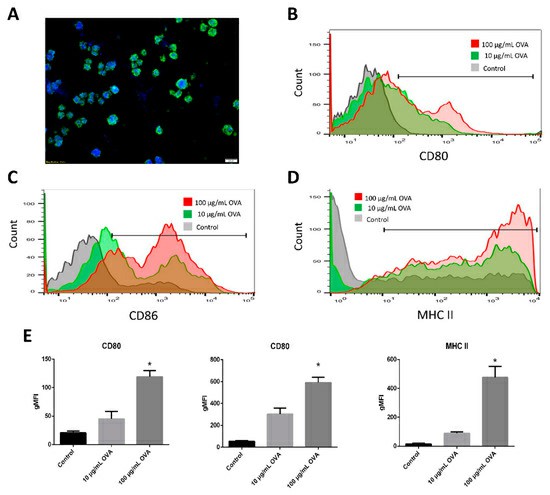 Fig. 1. Recellularization of bone marrow dendritic cells (BMDCs) into decellularized lymph node (dLN) scaffolds (Lin H, Wang W, et al., 2019).
Fig. 1. Recellularization of bone marrow dendritic cells (BMDCs) into decellularized lymph node (dLN) scaffolds (Lin H, Wang W, et al., 2019).
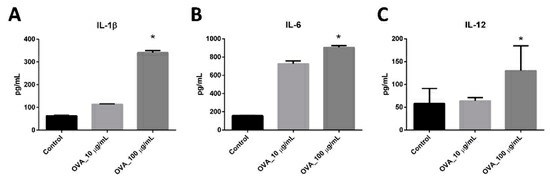 Fig. 2. The cytokine production of bone marrow dendritic cell decellularized lymph node scaffolds (BMDC-dLN) (Lin H, Wang W, et al., 2019).
Fig. 2. The cytokine production of bone marrow dendritic cell decellularized lymph node scaffolds (BMDC-dLN) (Lin H, Wang W, et al., 2019).
Expression of Surface Markers and Costimulatory Molecules by Stimulated DCs Ex Vivo
Baicalin, a compound extracted from the root of Scutellaria baicalensis, exhibits many anti-cancer effects, including immunomodulation and tumor inhibition. However, its solubility, weak absorption and low bioavailability prevent its clinical use. Wang's team set out to overcome these challenges by generating baicalin-engorged poly (lactic-co-glycolic acid) nanoparticles (PLGA-B).
Then, they investigated whether PLGA-B could simultaneously activate DCs and trigger cell death in melanoma cells, providing the basis for a new combination therapy combining immunotherapy and chemotherapy. Baicalin and PLGA-B promoted the maturation of bone marrow dendritic cells (BMDC) by incubating these compounds with DCs for 16 hours. Molecular expression of surface markers and costimulatory molecules was measured by flow cytometry, and cytokine production (IL-6, IL-12, TNF-α) was assessed by ELISA kits. CD40 and CD86 are critical for T-cell and B-cell activation and response. Lysosomal degradation for MHCII or cytoplasmic degrading for MHCI cross-presentation promotes cellular immunity (a key element of tumor immunotherapy). The immune activity of BMDCs was tested with baicalin and PLGA-B. PLGA-B at 2.68 g/mL, in particular, led DCs to display much higher levels of CD40, CD86, MHCI, and MHCII than free baicalin, even at high doses (Fig. 3). Thus, PLGA-B significantly improves baicalin's DC activation capacity at far lower levels
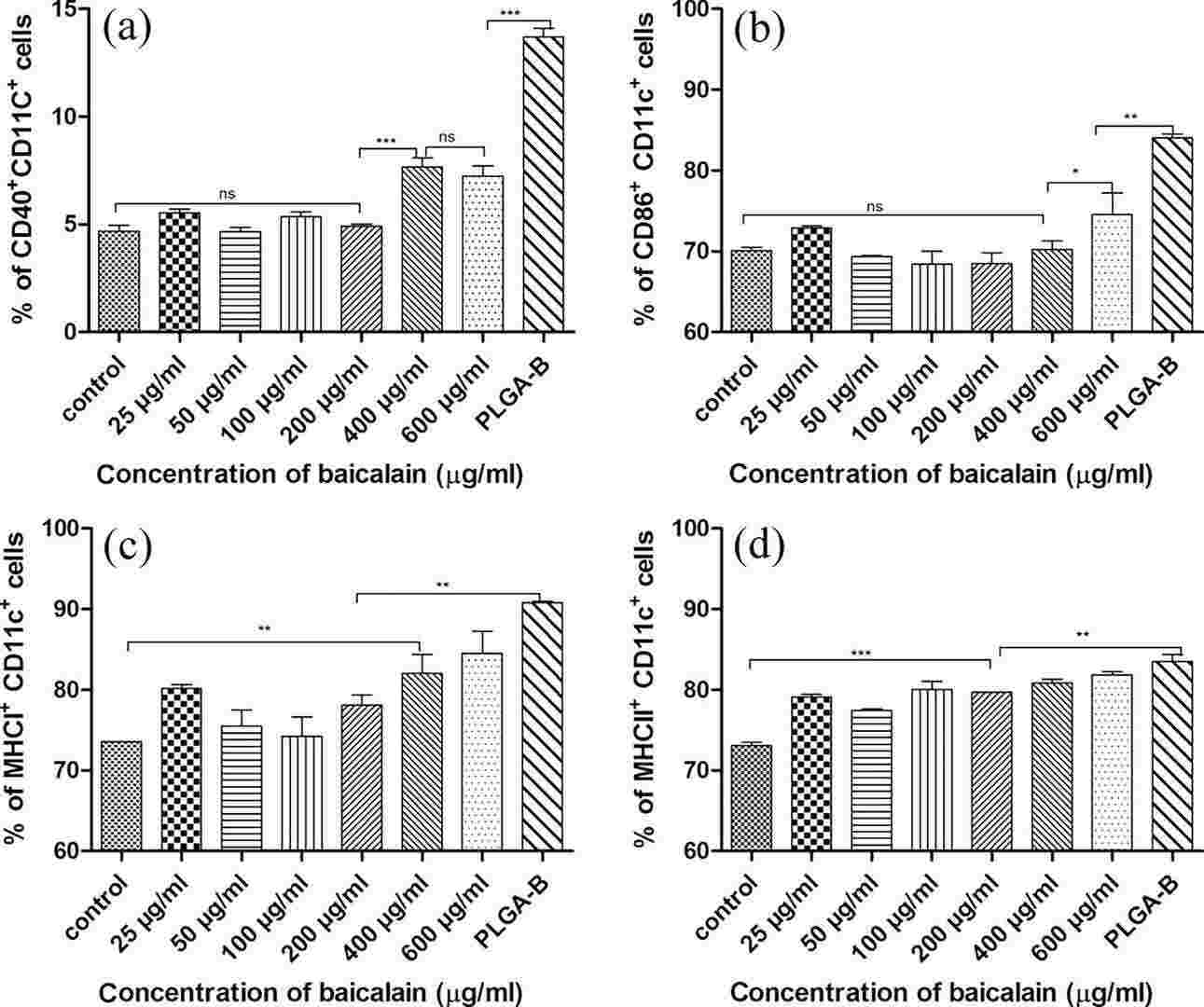 Fig. 3. Maturation and activation of dendritic cells (DCs) stimulated with baicalin and PLGA-B nanoparticles in vitro, showing the expression of the costimulatory molecules CD40 (a) CD86 (b), and surface markers MHCI (c) MHCII (d) (Wang H, Han S, et al., 2018).
Fig. 3. Maturation and activation of dendritic cells (DCs) stimulated with baicalin and PLGA-B nanoparticles in vitro, showing the expression of the costimulatory molecules CD40 (a) CD86 (b), and surface markers MHCI (c) MHCII (d) (Wang H, Han S, et al., 2018).
Ask a Question
Write your own review
- You May Also Need


