ONLINE INQUIRY
BALB/c Mouse Bone Marrow Dendritic Cells
Cat.No.: CSC-C4970J
Species: Mouse
Source: Bone Marrow
Cell Type: Dendritic Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can cryopreserved cells be kept at -20 °C.
Bone marrow dendritic cells (BMDCs) from BALB/c mice are isolated from the tibia and femur bone marrow of this mouse strain. Because BALB/c mice are a very common inbred, biomedical mouse strain, they are an ideal animal to develop vaccines, learn about the immune system, and test for toxicity of drugs, as they're extremely susceptible to various germs and medications.
Dendritic cells have branching or dendritic morphology. In their immature stage, they possess fewer cytoplasmic projections; as they mature, these numbers multiply, facilitating well cross-talk with T cells. During the immature state, bone marrow dendritic cells live mainly in the peripheral tissues, patrolling for potential pathogens and antigens. These cells are capable of phagocytosing an antigen and they will differentiate into adult dendritic cells upon incubation or stimulation with the antigen. Co-stimulatory and adhesion molecules are expressed less extensively in immature DCs than in adult DCs. BALB/c mouse bone marrow dendritic cells have become the essential tools of immunology research, serving as a base to develop vaccines, construct autoimmune disease models and investigate immune tolerance in immunology.
 Fig. 1. Mouse bone-marrow dendritic cell (Engevik MA, Ruan W, et al., 2021).
Fig. 1. Mouse bone-marrow dendritic cell (Engevik MA, Ruan W, et al., 2021).
Stimulating 1 ng/mL SEB Could Induce the Differentiation of Th2 and Treg Cells in Co-Culture with BMDCs
Staphylococcus aureus enterotoxin B (SEB) is a superantigen associated with food poisoning and immune diseases. It can bypass normal immune activation mechanisms, leading to atypical immune responses. Previous studies have shown SEB can stimulate different Th cell pathways but have not clarified the effects of dose variation.
Yuan' team co-cultured bone marrow dendritic cells (BMDCs) with naïve Th cells exposed to varying SEB doses (1, 10, and 100 ng/mL) and assessed expression levels of differentiation markers like T-bet and GATA-3, characterizing the differentiations of naïve Th cells stimulated with different doses of SEB. The gating strategy of Th cells is shown in Figure 1A. The results showed that a higher dose of SEB (100 ng/mL) increased T-bet expression and IFN-γ secretion in CD4 T cells. Both middle (10 ng/mL) and high doses (100 ng/mL) of SEB raised GATA-3 levels compared to the control (Fig. 2A and B). Th2/Th1 ratio was elevated in the 1 and 10 ng/mL groups (Fig. 2C). Th2 cytokines IL-4, IL-5, and IL-13 were up-regulated in all SEB groups (Fig. 2E-I). Tregs and IL-10 secretion significantly increased under SEB stimulation (Fig. 2G).
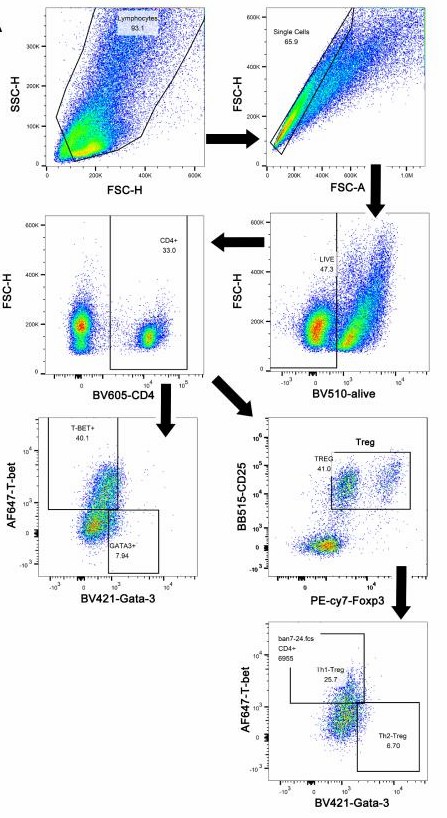 Fig. 1. The gating strategy of Th cells (Yuan J, Xu X, et al., 2023).
Fig. 1. The gating strategy of Th cells (Yuan J, Xu X, et al., 2023).
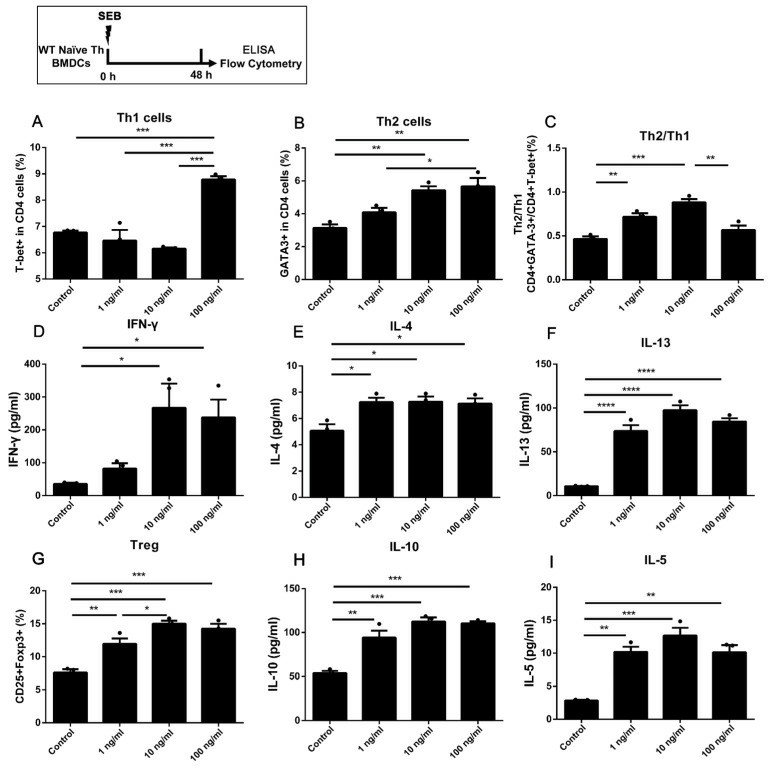 Fig. 2. The differentiation in WT naïve Th cells co-cultured with BMDCs under stimulation of SEB (Yuan J, Xu X, et al., 2023).
Fig. 2. The differentiation in WT naïve Th cells co-cultured with BMDCs under stimulation of SEB (Yuan J, Xu X, et al., 2023).
Immunomodulatory Effect of PNPS-0.3 on BMDCs
Panax notoginseng, a traditional Chinese medicine, is widely used for various health conditions yet leaves behind polysaccharide-rich residues post-extraction. Panax notoginseng polysaccharides (PNPS) have shown promising immunomodulatory effects with minimal side effects, making them potential immune response modifiers. Liu's team isolated and characterized a novel polysaccharide (PNPS-0.3) from the residue of Panax notoginseng by gradient elution, and then examined the immunomodulatory activities on the maturation of murine bone-marrow-derived dendritic cells (BMDCs).
Before the experiment, PNPS-0.3 and its more purified form, PNPS-0.3P, were incubated with BMDCs to measure CD80 expression using flow cytometry. Results showed no LPS contamination, with PNPS-0.3 causing stimulation, making it suitable for further evaluation as an immune adjuvant. Dosages of 12.5, 25, and 50 μg/ml were selected to study dosage effects. Flow cytometry measured expressions of surface markers CD40, CD80, CD86, and MHC II. PNPS increased these markers in a dose-dependent way (Fig. 3a). In the high dosage group (PNPS-0.3-H), expressions were significantly elevated compared to the blank. These expressions were also higher than in the LPS-treated control group. Fig. 3b displays BMDCs' morphologies. Untreated cells had a slightly rough surface with small dendrites whereas PNPS-0.3 notably altered BMDCs' shape, making them larger with oval or irregularly-shaped nuclei. The PNPS-0.3-H group showed more distinct features with longer protrusions and more folds. After 24 hours of exposure to PNPS-0.3, cytokines IL-12 and TNF-α levels were tested using ELISA kits. As depicted in Fig. 3c, PNPS-0.3 enhanced cytokine secretion dose-dependently. The PNPS-0.3-H group showed the highest IL-12 and TNF-α levels, significantly exceeding those of the blank group and the control group.
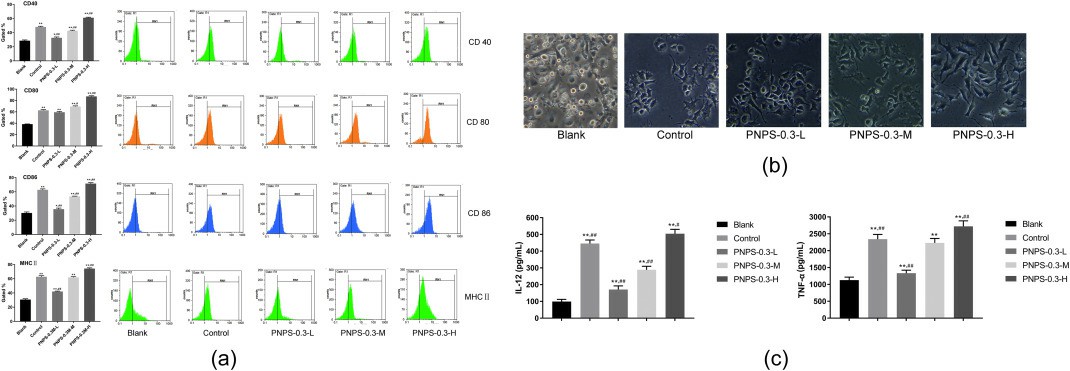 Fig. 3. Immunomodulatory effect of PNPS-03 on BMDCs (Liu S, Yang Y, et al., 2020).
Fig. 3. Immunomodulatory effect of PNPS-03 on BMDCs (Liu S, Yang Y, et al., 2020).
Ask a Question
Write your own review
- You May Also Need