ONLINE INQUIRY
Rat Skeletal Muscle Cells
Cat.No.: CSC-C9387W
Species: Rat
Source: Skeletal Muscle
Morphology: Multipolar
Cell Type: Skeletal Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Skeletal Muscle Cells originate from healthy muscle of rats. The skeletal muscle cells, or striated muscle cells, are some of the largest cells in human and animal bodies. They have lots of myofibrils that align along the cell's long axis with periodic striations, and are what makes skeletal muscle cells so distinct from other cells. They are multinucleated cells, assembled from myoblast fusion, so the assembly of skeletal muscle is a complicated process that requires multiple signaling pathways: phosphatidylinositol 3-kinase, calcineurin, STAT3, MAPK. These are specialized, functional cells that can scaffold, bind, shield and nourish muscles. They will fusion into multinucleated myotubes and striated muscle fibers under culture conditions, the extent of which will decrease with serial passages.
Under normal physiological conditions in rats, the skeletal muscle cells have one task: body movement and posture. Additionally, skeletal muscle is an important metabolic organ and plays multiple metabolic roles, such as metabolizing carbohydrates, fats and proteins. For research, rat skeletal muscle cells are frequently employed in muscle biology, exercise physiology, regenerative medicine and drug testing. They are, for instance, largely employed to examine the repair of muscle injury, the mechanism of muscle atrophy with age, and the use of drugs or gene therapies for muscle disease.
 Fig. 1. Rat skeletal muscle cell cultures (Mortensen O H, Frandsen L, et al., 2006).
Fig. 1. Rat skeletal muscle cell cultures (Mortensen O H, Frandsen L, et al., 2006).
Protective Effect of Luffa cylindrica Roemer Against Dexamethasone-Induced Muscle Atrophy in Primary Rat Skeletal Muscle Cells
Glucocorticoids (GCs), commonly prescribed for chronic inflammatory conditions, can cause muscle atrophy (MA), reducing quality of life and increasing mortality. MA involves protein degradation instigated by GCs like dexamethasone, resulting in muscle mass loss. High doses prolong catabolic effects, activating E3 ubiquitin ligases. Studies often explore plant extracts for mitigating MA effects. Previous studies have reported that Luffa cylindrica Roemer (LCR) contains gallic acid and uritin B are effective for muscle atrophy.
Yeo's team investigated LCR's anti-MA potential using a DEX-treated primary rat skeletal muscle cell model, assessing changes in myotube development, viability, and MA markers to explore its therapeutic efficacy and safety. Initially, primary myoblasts were harvested from the tibialis anterior muscle of 1-day-old rats and induced to differentiate into myotubes, during which spontaneous beating and myotube-like structures formed. They conducted a CCK-8 assay to assess LCR's cytotoxicity on differentiated myotubes. LCR treatment ranging from 1 to 400 μg/ml significantly enhanced cell viability at >25 μg/ml (Fig. 1a). To examine LCR's effect during DEX-induced atrophy, primary myotubes were treated with 50, 100, and 200 μM DEX, based on previous studies (Wang et al. 2021) (Fig. 1b). We noted no significant diameter difference at 50, 100 μM, but a significant reduction at 200 μM DEX (Fig. 1c), establishing it as our MA model. DEX alone decreased cell viability, whereas LCR co-treatment restored it dose-dependently (Fig. 1d). Results indicate LCR is non-toxic and offers protective effects against DEX-induced MA.
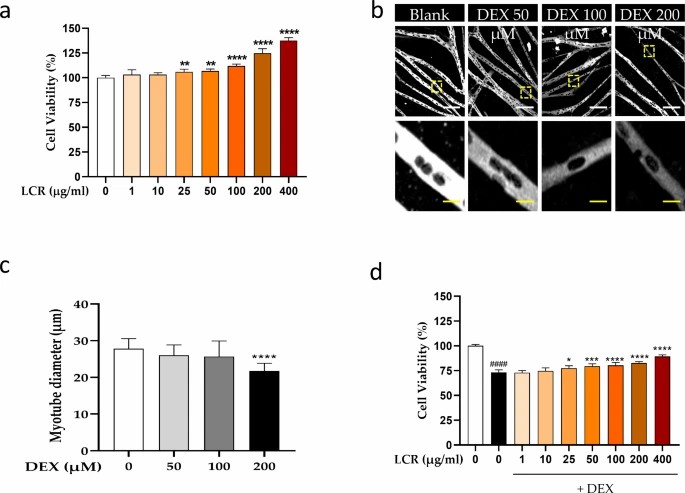 Fig. 1. Potential protective effect of Luffa cylindrica Roemer (LCR) by increasing cell viability in primary myotubes (Yeo C, Kim H, et al., 2024).
Fig. 1. Potential protective effect of Luffa cylindrica Roemer (LCR) by increasing cell viability in primary myotubes (Yeo C, Kim H, et al., 2024).
Micropatterning and Alignment of C2C12 Myoblast and Primary Skeletal Muscle Cells Using Microflowed Plasma Process
Understanding and controlling the orientation and behavior of skeletal muscle cells is crucial for studying cellular processes and mimicking physiological conditions. Traditional surfaces fail to replicate the in vivo environment due to lack of structural cues. Micropatterning emerges as a strategy to create environments resembling tissue conditions, aiding in studying cell behavior. Vajanthri's team aimed to optimize micropatterning environments using the microchannel flowed plasma process, creating specific adhesive widths on glass substrates to guide the alignment and orientation of skeletal myoblast cells effectively.
The primary skeletal muscle cells stained with PAX7 antibody confirmed their stem cell/satellite origin, as shown in Fig. 2. Bright field microscopy was used to observe myoblast alignment on micropatterned glass substrates. Both C2C12 and primary skeletal muscle cells were cultured on OTS/APTES modified coverslips with cell adhesive region widths of 20 μm, 200 μm, and 1000 μm (Fig. 3A-C). The images reveal myoblast alignment within adhesive regions, while methyl-terminated SAMs inhibited adhesion. Over 5 and 8 days, cells remained aligned within adhesive regions despite decreasing widths. Initially, alignment began near boundaries, propagating inward with culture progression. Such dynamics, especially at interfaces, were consistent with previous studies and highlighted in broader areas (Fig. 3C), suggesting initial alignment influences neighbors.
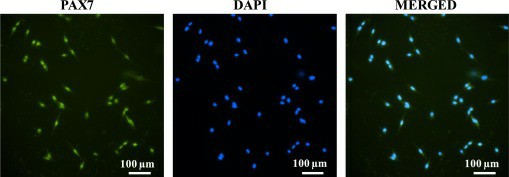 Fig. 2. Fluorescent images of isolated cells stained with anti PAX7 antibody (green-PAX7 transcription factor), nuclei stained with DAPI (blue-nuclei) and merged image (Vajanthria K Y, Sidu R K., et al., 2020).
Fig. 2. Fluorescent images of isolated cells stained with anti PAX7 antibody (green-PAX7 transcription factor), nuclei stained with DAPI (blue-nuclei) and merged image (Vajanthria K Y, Sidu R K., et al., 2020).
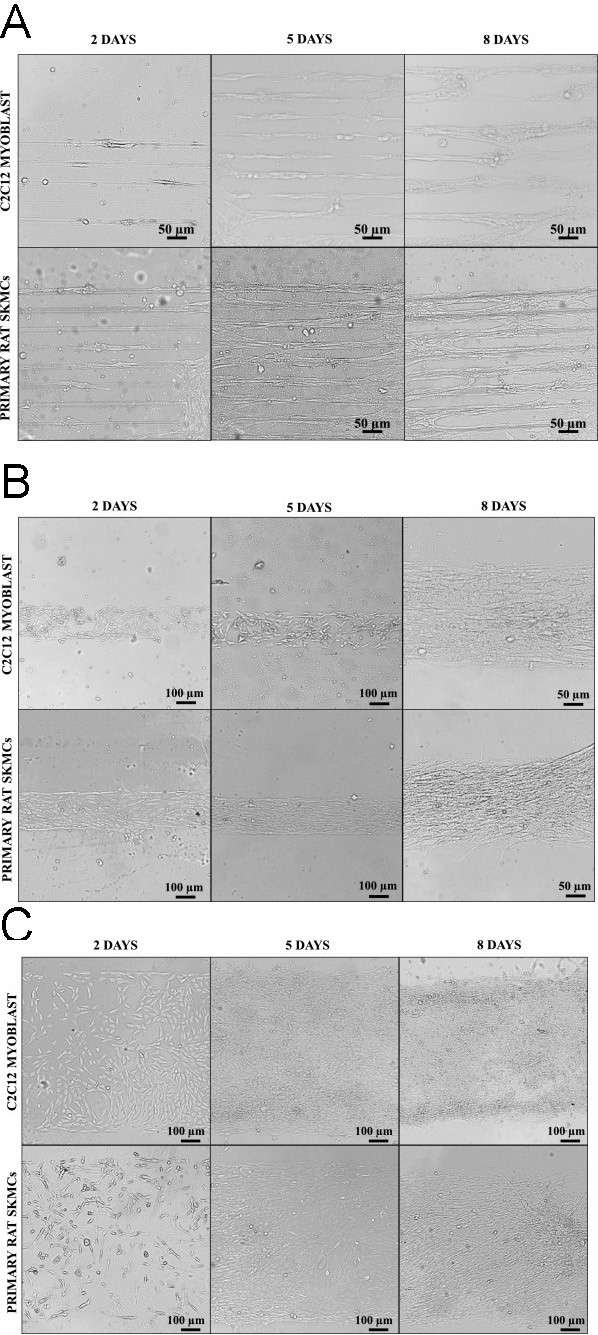 Fig. 3. Bright-field images of C2C12 and primary rat skeletal muscle cells cultured on cell adhesive width of 20 μm (A), 200 μm (B), and 1000 μm (C) for 2, 5 and 8 days (Vajanthria K Y, Sidu R K., et al., 2020).
Fig. 3. Bright-field images of C2C12 and primary rat skeletal muscle cells cultured on cell adhesive width of 20 μm (A), 200 μm (B), and 1000 μm (C) for 2, 5 and 8 days (Vajanthria K Y, Sidu R K., et al., 2020).
Ask a Question
Write your own review