ONLINE INQUIRY
Rat Pulmonary Microvascular Endothelial Cells
Cat.No.: CSC-C9359W
Species: Rat
Source: Lung
Morphology: polyglonal
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
In rats, pulmonary microvascular endothelial cells (PMVECs) can be found on the inner surface of the pulmonary artery walls, where they form a single-cell layer glued to the wall. When monolayered, these spindle-shaped or polygonal cells take a normal cobblestone or pavement shape. As a key component of the blood-air barrier, PMVECs regulate the dynamical interplay between the inner and outer lining of blood vessels, the integrity of the pulmonary interior, and the interplay between coagulation and fibrinolysis. They also have endocrine and metabolic functions, which enable them to make and release various bioactive compounds, including angiotensin-converting enzyme, which plays a role in maintaining vascular tone and blood pressure.
In vitro, PMVECs can form tubular structures mimicking angiogenesis. Studies have demonstrated that PMVECs have powerful anti-apoptotic activity in pathological conditions, like pulmonary ischemia-reperfusion injury (IRI). In addition, PMVECs can develop functional vessels by implanting collagen gels in vivo. It's also possible to use PMVECs to create cell models for other lung disorders including pulmonary fibrosis, pulmonary edema, acute lung injury, and pulmonary arterial hypertension. These features render PMVECs an important tool for investigating the pathogenesis of lung disorders and identifying novel therapeutic targets.
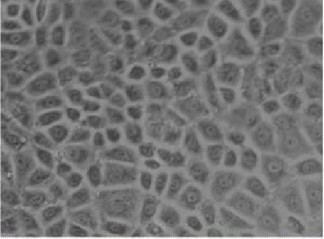 Fig. 1. Rat pulmonary microvascular endothelial cells (RPMVECs) morphology (Chun C, Liu H, et al., 2007).
Fig. 1. Rat pulmonary microvascular endothelial cells (RPMVECs) morphology (Chun C, Liu H, et al., 2007).
STS Prevents the Hypoxic Downregulation of BMPR2-Smad1/5/9 Signaling Pathway in the PA Endothelium in PMVECs
Pulmonary hypertension (PH) features substantial vascular remodeling and heightened pulmonary arterial pressure, leading to severe cardiac strain. Existing treatments are costly and not fully effective, prompting the exploration of alternatives like sodium tanshinone IIA sulfonate (STS). STS has shown potential against PH by modulating ion channels in smooth muscle cells, but its role in endothelial health is understudied. Using a hypoxia-induced PH rat model and cultured rat pulmonary microvascular endothelial cells (PMVECs), Wang's team examined STS's effects on the BMP9-BMPR2-Smad1/5/9 signaling pathway.
Experiments in rat models showed that STS treatment prevents the HPH disease development and significantly prevents the hypoxia-induced apoptosis of the PA endothelium. Then, they examined the effects of STS on the BMP9-BMPR2-Smad1/5/9 pathway. As shown in Fig. 1, HPH rats exhibited reduced levels of BMPR2, p-Smad1/5/9, and Caveolin-1 compared to normoxic controls. STS treatment prevented these reductions without affecting the normoxic group. Notably, while ALK1 levels increased under hypoxia, STS had little effect on this receptor. These findings were confirmed in PMVECs exposed to hypoxia (4% O2, 72-h) with or without STS (12.5 µM) (Fig. 2). STS did not alter BMPR2 transcription, suggesting its impact occurs at the protein degradation level. Using CHX, STS delayed BMPR2 protein degradation in hypoxic PMVECs, especially when combined with lysosome inhibitor CHQ, but not with UPS inhibitor MG132 (Fig. 3). STS also enhanced BMPR2's membrane localization and interaction with Caveolin-1, highlighting STS's role in stabilizing BMPR2.
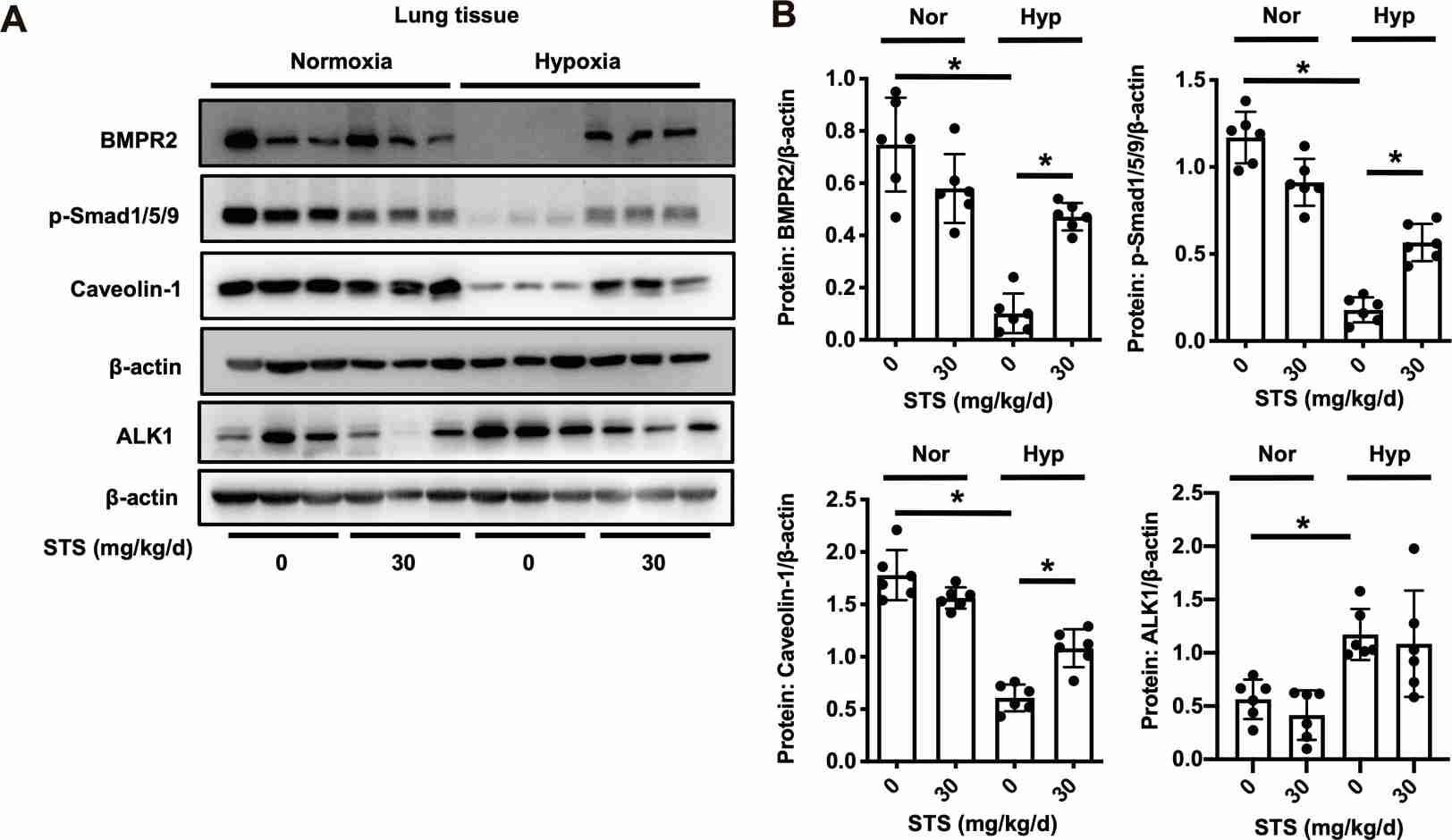 Fig. 1. STS treatment prevents the hypoxic downregulation of BMPR2 signaling pathway in the lung tissue (Wang J, Liu W, et al., 2022).
Fig. 1. STS treatment prevents the hypoxic downregulation of BMPR2 signaling pathway in the lung tissue (Wang J, Liu W, et al., 2022).
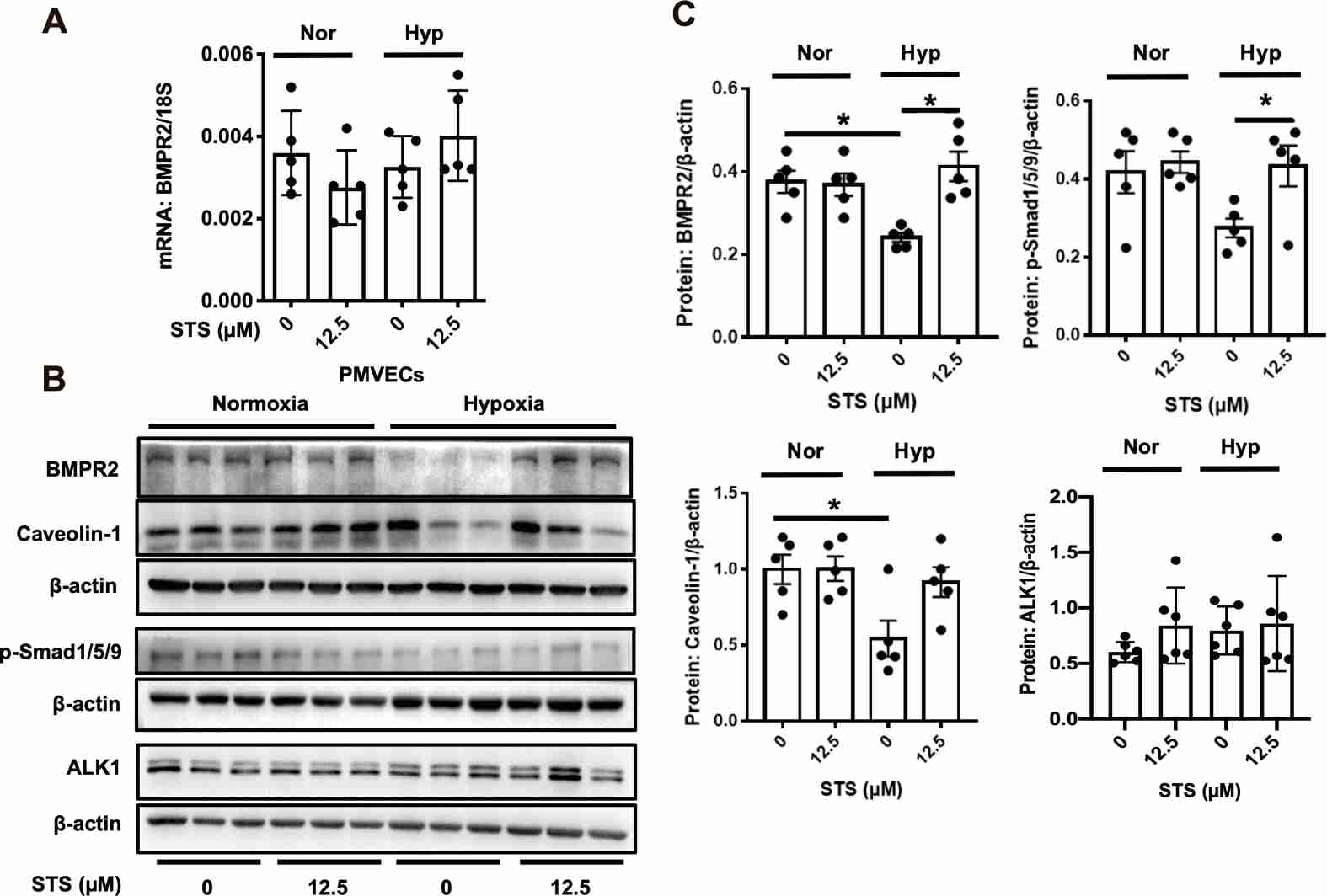 Fig. 2. STS treatment prevents the hypoxic downregulation of BMPR2 signaling pathway in primarily cultured rat PMVECs (Wang J, Liu W, et al., 2022).
Fig. 2. STS treatment prevents the hypoxic downregulation of BMPR2 signaling pathway in primarily cultured rat PMVECs (Wang J, Liu W, et al., 2022).
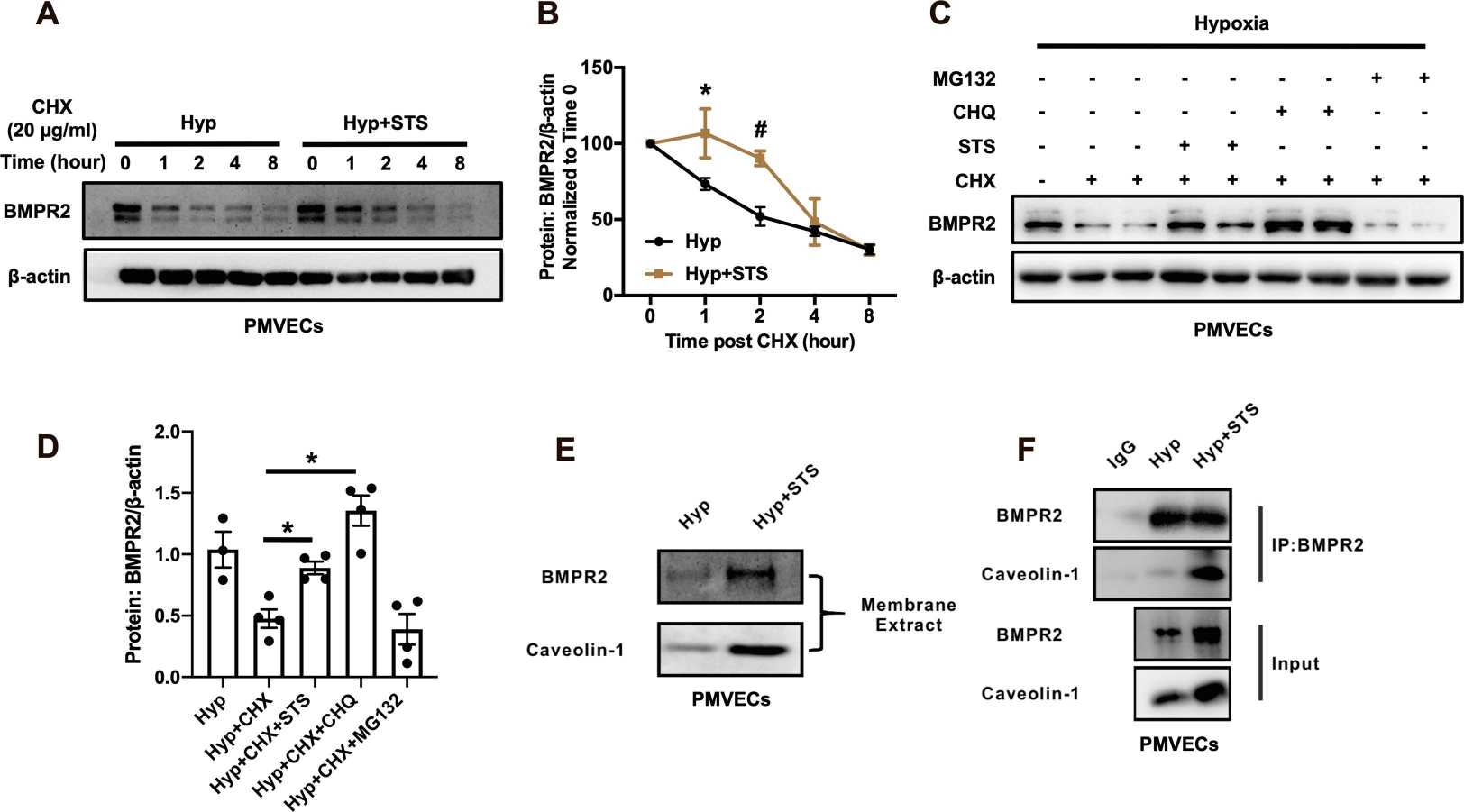 Fig. 3. STS significantly increases the BMPR2 protein stability and inhibits its lysosomal degradation in hypoxic rat PMVECs (Wang J, Liu W, et al., 2022).
Fig. 3. STS significantly increases the BMPR2 protein stability and inhibits its lysosomal degradation in hypoxic rat PMVECs (Wang J, Liu W, et al., 2022).
Fasudil Inhibited LPS-Induced Inflammatory Factors Expression in Rat PMVECs
Inflammatory conditions like pulmonary artery hypertension (PAH) and the acute lung injury (ALI) are marked by PMVEC dysfunction due to factors like trauma or infection. Fasudil, a Rho kinase (ROCK) inhibitor, has shown promise in treating PAH with anti-inflammatory effects. Liu's team investigated fasudil's effect on lipopolysaccharide (LPS)-induced inflammatory damage in rat PMVECs, focusing on its capacity to mitigate inflammation and oxidative stress by modulating the ROS-NF-κB signaling pathway.
To assess Fasudil's protective effects against LPS-induced inflammation, the mRNA levels of IL-6, TNF-α, and MCP-1 were measured in rat PMVECs. As shown in Fig. 4A and B, LPS exposure significantly increased MCP-1 and IL-6 mRNA, peaking at 6 and 12 hours, respectively, while TNF-α remained unchanged. Pretreatment with fasudil significantly reduced these mRNA levels in a concentration-dependent manner (Fig. 4C and D). These results suggested that fasudil effectively inhibited LPS-induced inflammation response in rat PMVECs. Additionally, ELISA results showed increased IL-6 and MCP-1 protein levels in LPS-treated samples (Fig. 4E and F), which fasudil also effectively reduced. These results further confirmed that fasudil could inhibit the inflammatory response induced by LPS in rat PMVECs.
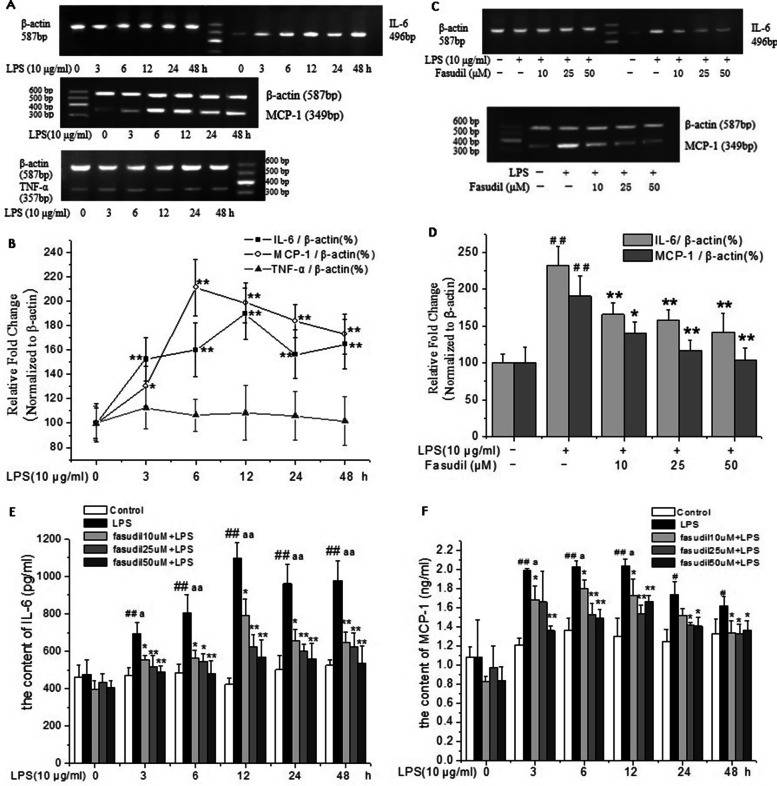 Fig. 4. Fasudil inhibited LPS-induced inflammatory factors expression in rat PMVECs (Liu H, Pan Z, et al., 2022).
Fig. 4. Fasudil inhibited LPS-induced inflammatory factors expression in rat PMVECs (Liu H, Pan Z, et al., 2022).
Ask a Question
Write your own review