Rat Podocytes
Cat.No.: CSC-C9378W
Species: Rat
Source: Kidney
Morphology: Epithelial-like
Cell Type: Podocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat podocytes, a primary glomerular cell type of rat kidney podocyte lines, have been used extensively as a model for kidney function and pathology. In the glomerulus, these visceral epithelial cells attach to the basement membrane surrounding the capillaries via branching protrusions in a sophisticated tertiary "foot" process network. These processes bundle together to make a slit diaphragm, which prevents the accumulation of proteins and other large molecules in the urine, keeping the structure of the glomerular filtration barrier intact.
The cytoskeleton of podocytes is rich in actin, allowing them to dynamically remodel and adapt to pressure changes. Their complex shape and molecular dynamics keep the flux of substances between blood and urine exactly under control, maintaining the internal environment. In immunofluorescence testing, podocyte markers can be identified including podocin, angiotensin 1, nephrin, actin 4, and NPHS2. In clinical research, rat podocyte lines play an essential role in the study of glomerular disease, especially diabetic nephropathy and membranous nephropathy. The lineages of in vitro cultured podocytes offer a robust model for understanding podocyte biology, molecular biology and drug discovery. They are also critical for examining cell signaling pathways, mechanisms of podocyte damage and therapeutic strategies, helping to uncover the underlying pathology of renal disease and experimentally supporting new therapies.
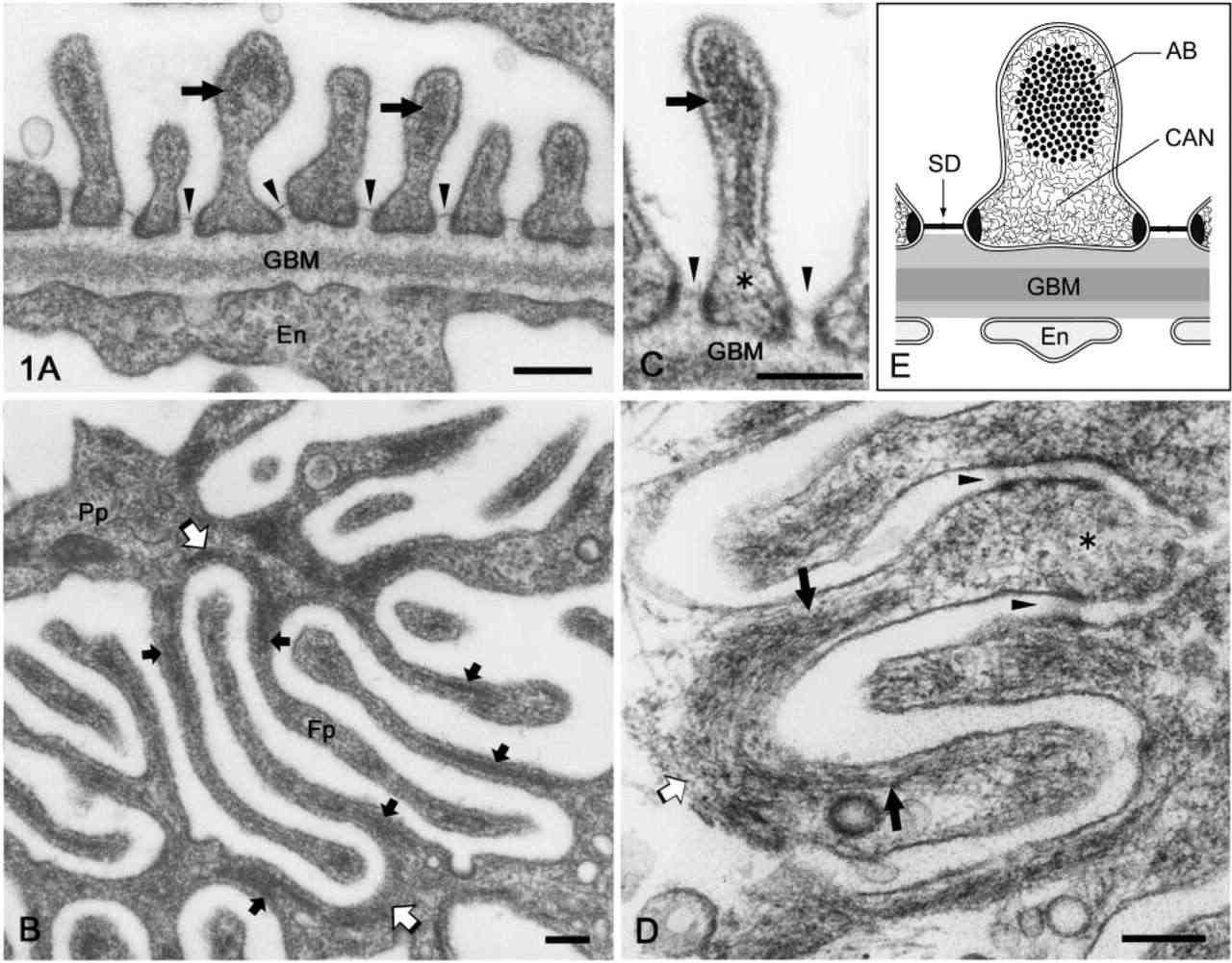 Fig. 1. Electron micrographs of rat podocyte foot processes. (a) Perpendicular sections. (b, d) Horizontal sections. (c) Cross-sections (Ichimura K, Kurihara H, et al., 2003).
Fig. 1. Electron micrographs of rat podocyte foot processes. (a) Perpendicular sections. (b, d) Horizontal sections. (c) Cross-sections (Ichimura K, Kurihara H, et al., 2003).
Acute Glucose Fluctuation Promotes Autophagy and IL-1β and TNF-α Generation in Rat Podocytes
Diabetic nephropathy (DN) is a serious diabetes-related kidney disease in which acute glucose fluctuation (AGF) is one risk factor that can lead to greater kidney damage than steady-state hyperglycemia. But the exact mechanisms are not clear. Hu's team is trying to find out how AGF affects the progression of DN. They used rat podocytes cultured under 72-hour AGF to monitor cell proliferation, apoptosis, oxidative stress and autophagy.
CCK-8 showed a reduced viability of cells in high‑glucose group (HG) compared with normal glucose group (NG), and also in AGF group compared with both NG and HG group (Fig. 1B). Rates of apoptosis were not significantly different between groups (Fig. 1C and D). Autophagy, a major component of podocytes, was measured by western blot analysis of autophagy biomarkers (LC3II/I and Beclin-1). AGF treatment significantly elevated LC3II/I and Beclin-1, while the NG and HG group showed a decrease (Fig. 2A and B). Confocal microscopy demonstrated a stronger presence of yellow and red puncta in AGF compared with HG (Fig. 2C and D). In order to test whether AGF influences inflammation, mRNA and protein concentrations of TNF-α and interleukin-1β (IL-1β) were measured in the treated rat podocytes. Both cytokines were higher in the HG and AGF groups than in the NG group, and both were substantially higher in the AGF group than in the HG group (Fig. 3). This indicates that AGF promotes inflammatory response in rat podocytes.
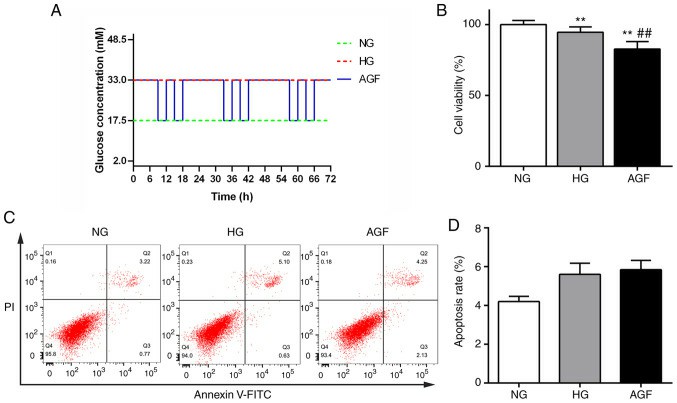 Fig. 1. Effect of acute blood fluctuation on rat podocyte proliferation and apoptosis (Hu Z, Fang W, et al., 2021).
Fig. 1. Effect of acute blood fluctuation on rat podocyte proliferation and apoptosis (Hu Z, Fang W, et al., 2021).
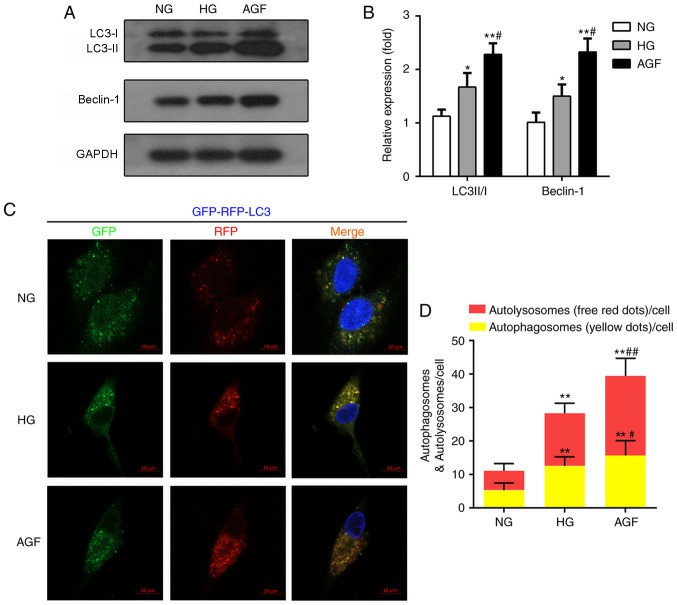 Fig. 2. AGF induces autophagy in rat podocytes (Hu Z, Fang W, et al., 2021).
Fig. 2. AGF induces autophagy in rat podocytes (Hu Z, Fang W, et al., 2021).
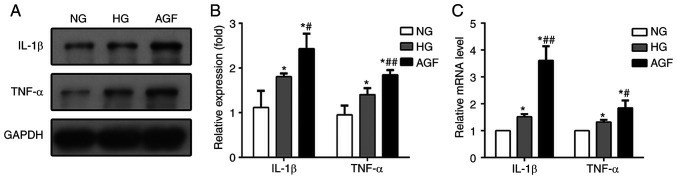 Fig. 3. AGF promotes pro-inflammatory cytokine generation (Hu Z, Fang W, et al., 2021).
Fig. 3. AGF promotes pro-inflammatory cytokine generation (Hu Z, Fang W, et al., 2021).
Rac1 and RhoA Activity Influences Rat Podocyte Permeability and Migration
Podocyte foot processes maintain glomerular permeability and are regulated by insulin through the PKGIα signaling pathway. Disruption of this mechanism in diabetes impacts the actin cytoskeleton, which depends on Rho GTPases. Rachubik et al. used Rho GTPases inhibitors and siRNA in cultured cells and Zucker rats to explored PKGIα-Rac1-RhoA's role in podocyte cytoskeletal organization.
Insulin significantly increased glomerular albumin permeability (Palb). This increase was reduced by Rac1 inhibitors EHT1864 and NSC23766 by 32% and 57%, respectively, (Fig. 4A). Conversely, RhoA inhibitors (Ripasudil acid and Y27632) increased Palb by 240% and didn't affect Palb with insulin. Similar trends were observed in podocyte layer albumin permeability (Fig. 4B). Insulin increased albumin flux by 197%, reduced in the presence of Rac1 inhibitors. RhoA inhibitors also elevated permeability through the podocyte monolayer. To investigate how small GTPases influence cytoskeleton dynamics and podocyte migration, they utilized NSC23766 and Y27632 inhibitors. Quantitative analysis showed that insulin increased F-actin near the plasma membrane (Fig. 5). They hypothesized that this insulin-driven cytoskeletal reorganization is Rac1-dependent, as preincubation with the Rac1 inhibitor NSC23666 lessened the effect. RhoA inhibition also mimicked insulin's effect (Fig. 5). Wound-healing assay revealed insulin boosted podocyte migration, blocked by NSC23766, but not by Y27632, indicating insulin's role as a key Rac1-dependent actin remodeling and migration regulator.
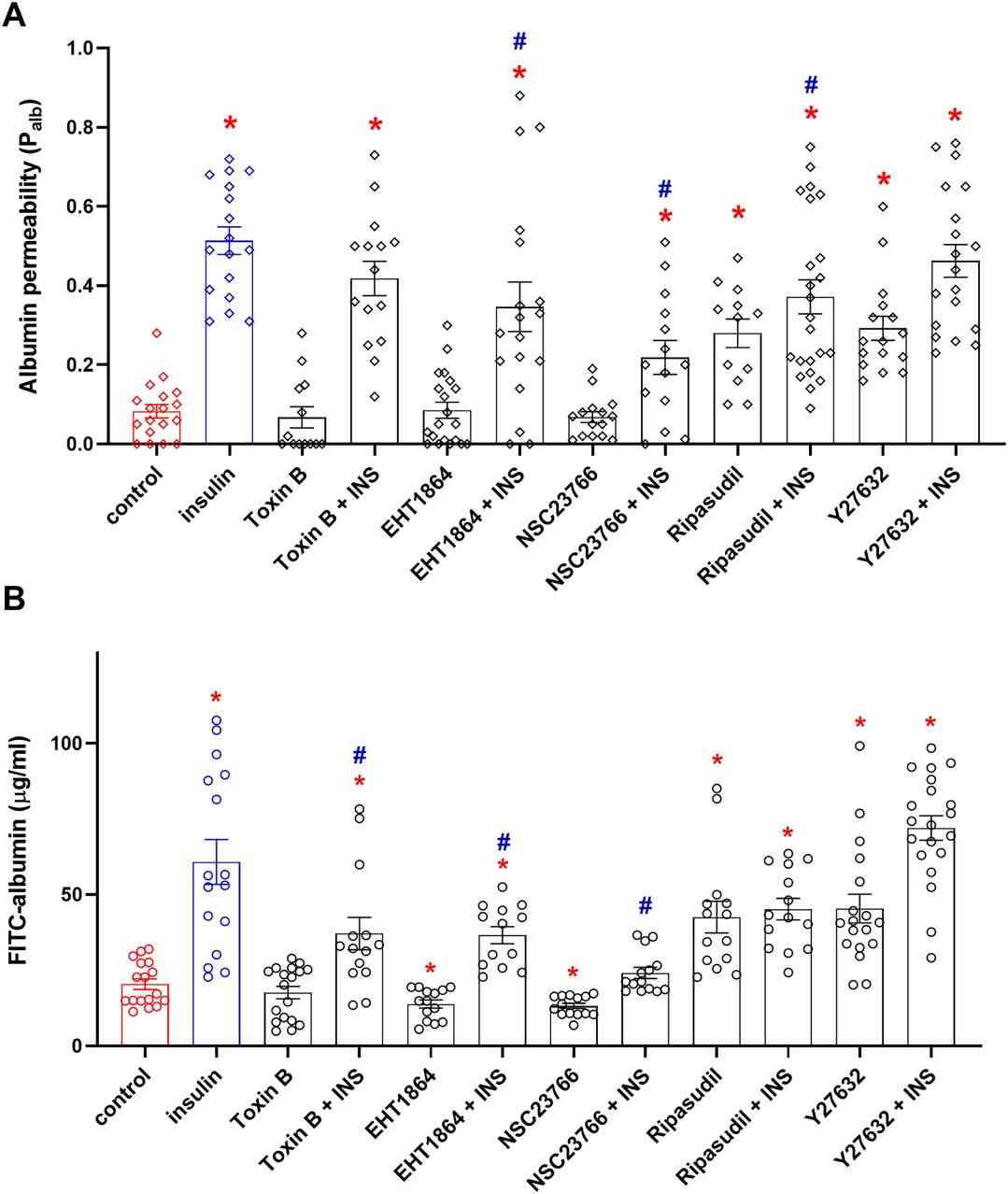 Fig. 4. Rho family GTPases regulate glomerular filtration barrier permeability (Rachubik R, Szrejder M, et al., 2022).
Fig. 4. Rho family GTPases regulate glomerular filtration barrier permeability (Rachubik R, Szrejder M, et al., 2022).
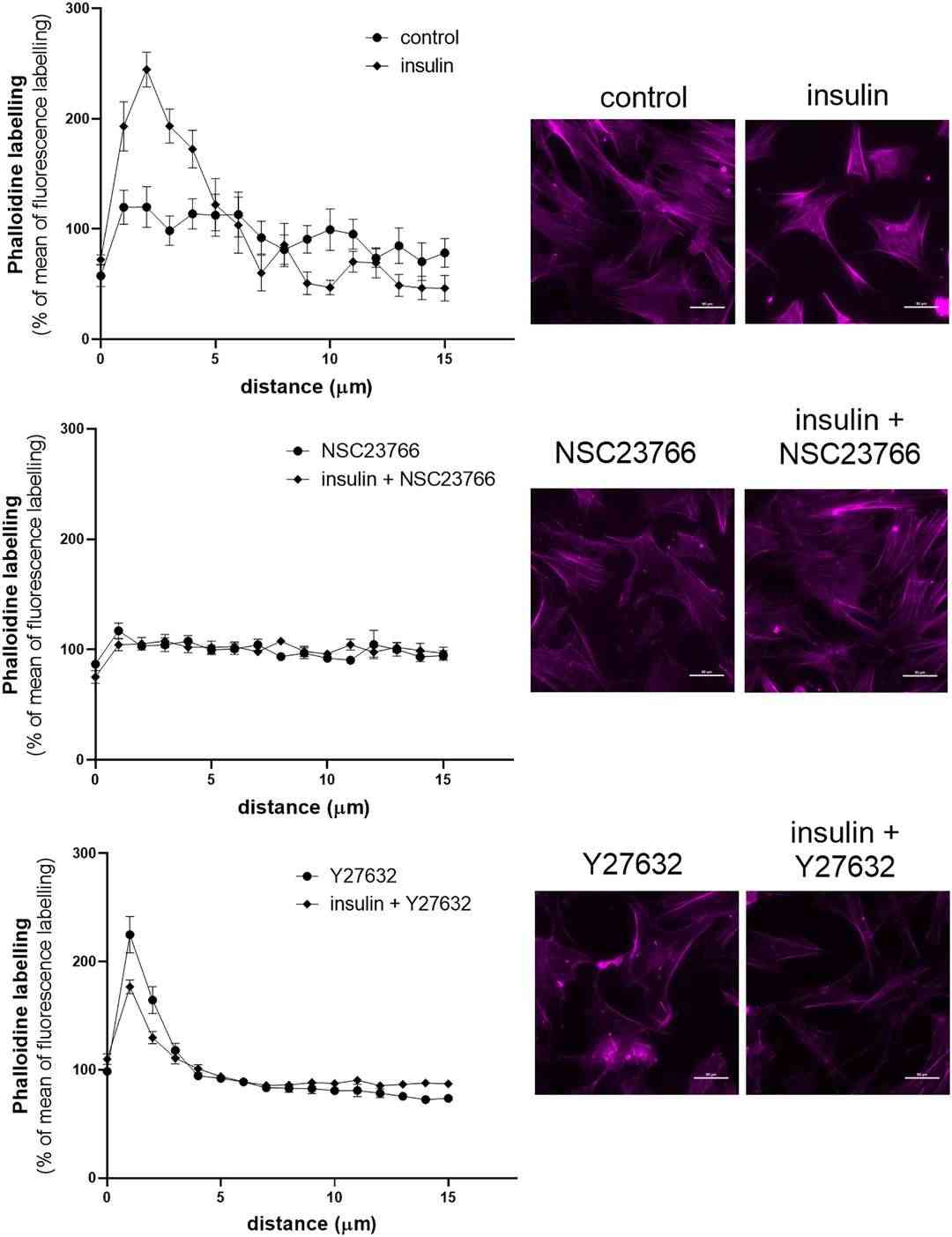 Fig. 5. Insulin redistributes F-actin to the cell periphery via Rac1 signaling pathway (Rachubik R, Szrejder M, et al., 2022).
Fig. 5. Insulin redistributes F-actin to the cell periphery via Rac1 signaling pathway (Rachubik R, Szrejder M, et al., 2022).
Ask a Question
Write your own review