ONLINE INQUIRY
Rat Astrocytes Cerebellar
Cat.No.: CSC-C4414X
Species: Rat
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Rat Astrocytes Cerebellar (RA-c) cells form spindle and polygonal structures, typical of astrocytes. These cells are highly distributed throughout the central nervous system, but especially in the cerebellar cortex where they are connected to neurons through tangled networks. Through long, branching processes, astrocytes plug the gaps between neurons, and thereby support and protect them. RA-c cells offer great scientific utility in many ways. For one, they are highly plastic, so they can accommodate diverse physiological and pathological contexts. Secondly, RA-c cells are capable of releasing various neurotrophic factors that promote neuronal growth, development and survival. They are also involved in synapse development and maintenance, and in the establishment and repair of the blood-brain barrier.
The RA-c cell line has broad applications in neuroscience research. They serve as essential tools for investigating the functions and characteristics of astrocytes. By cultivating and studying RA-c cells in vitro, scientists can gain deeper insights into the roles and mechanisms of astrocytes within the nervous system. Moreover, RA-c cells hold potential application value in the research of neurodegenerative diseases and brain injuries. By simulating pathological environments and studying functional changes in RA-c cells, researchers can develop new strategies and methods for treating neurodegenerative diseases.
MeHg-Induced Alterations in Cell Viability and Intracellular ROS Level on MeHg in Cultured Normal Rat Cerebellar Astrocytes
Methylmercury (MeHg), a widespread environmental pollutant, poses severe threats to the central nervous system, primarily through reactive oxygen species (ROS) induction and oxidative stress. Sasaki's team aimed to uncover the mechanisms of MeHg toxicity in astrocytes, focusing on the involvement of reactive oxygen species (ROS).
They explored MeHg toxicity in cultured normal rat cerebellar astrocytes (NRA), assessing cell viability and ROS levels. The cell viability of NRA simultaneously exposed to MeHg and/or three antioxidants, Trolox, N -acetyl-L-cysteine (NAC), and glutathione (GSH), was evaluated before analyze protein expression. MeHg exposure increased viability at specific concentrations (2.5 µM for 48 h, 1.25-2.5 µM for 96 h, and 0.625 µM for 144 h) but decreased it at higher concentrations (12.5-50 µM for 48 h, ≥ 5 µM for 96 h, and ≥ 2.5 µM for 144 h) (Fig. 1A). Trolox reduced the viability increase from 2 µM MeHg and mitigated viability loss from 4 µM MeHg (Fig. 1B). NAC similarly suppressed MeHg-induced viability changes (Fig. 1C). GSH showed minimal effect alone but decreased viability when combined with 2 µM MeHg and increased viability when combined with 4 µM MeHg (Fig. 1D). Intracellular ROS levels in NRA cells were measured using the ROS-sensitive fluorescent dye DCFDA/H2DCFDA. Exposure to 2 or 2.5 µM MeHg for 96 hours significantly increased intracellular ROS, while 5 or 10 µM MeHg decreased it (Fig. 2A). Trolox exposure significantly reduced ROS levels in NRA exposed to 2 µM and 4 µM MeHg and in control (0 µM) cells (Fig. 2B). NAC notably inhibited both the increase from 2 µM MeHg and the decrease from 4 µM MeHg in ROS levels (Fig. 2C). Contrarily, GSH enhanced ROS levels due to 2 µM MeHg and reversed the decrease by 5 µM MeHg, exceeding control levels (Fig. 2D). These findings showed that while NAC and Trolox mitigated some toxicity effects, glutathione exacerbated them. Thus, MeHg-induced ROS plays a key role in toxicity, paving the way for potential therapeutic approaches targeting astrocytes.
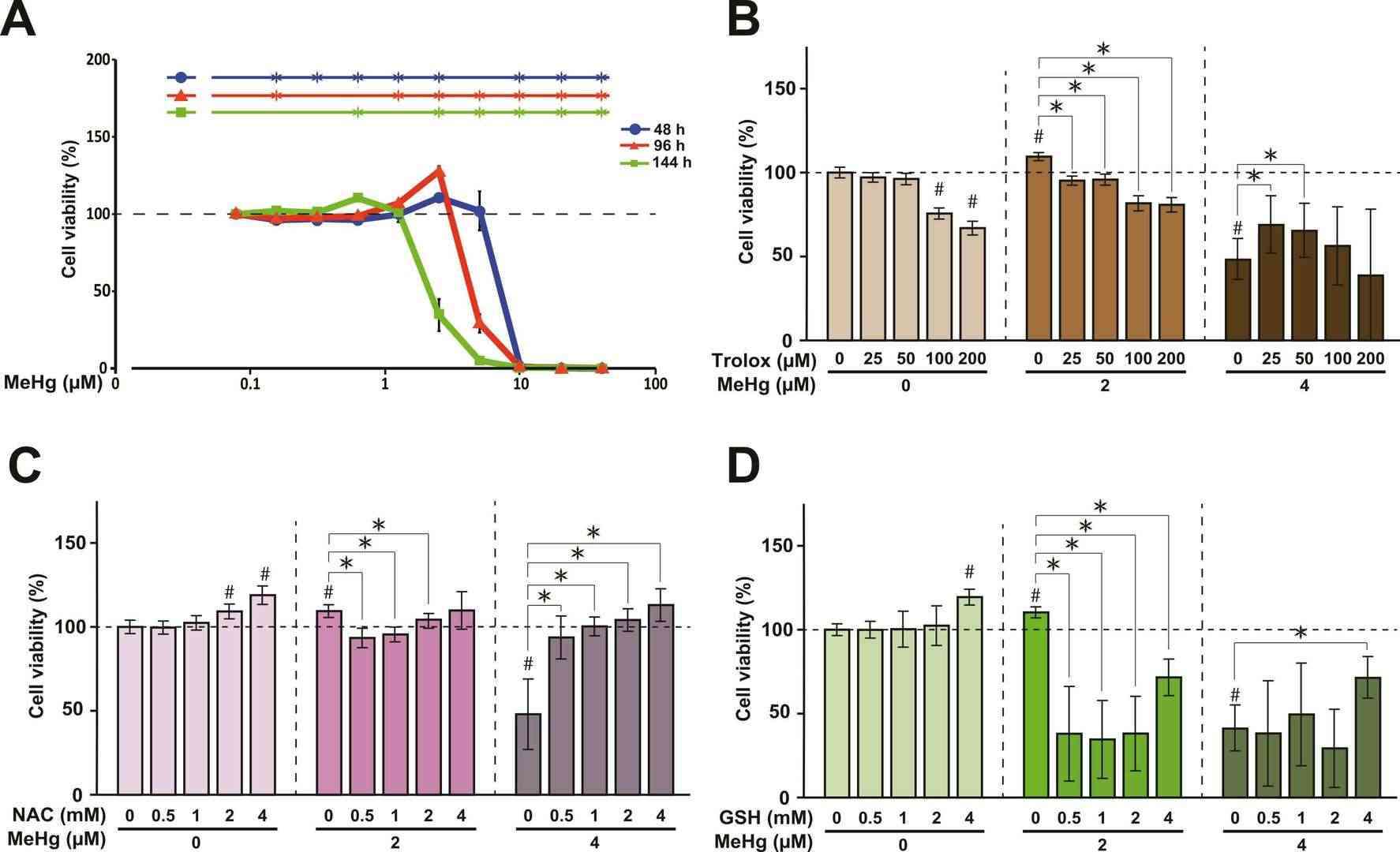 Fig. 1. Dose- and time-dependent alterations in cell viability in NRA exposed to MeHg (Sasaki S, Negishi T, et al., 2023).
Fig. 1. Dose- and time-dependent alterations in cell viability in NRA exposed to MeHg (Sasaki S, Negishi T, et al., 2023).
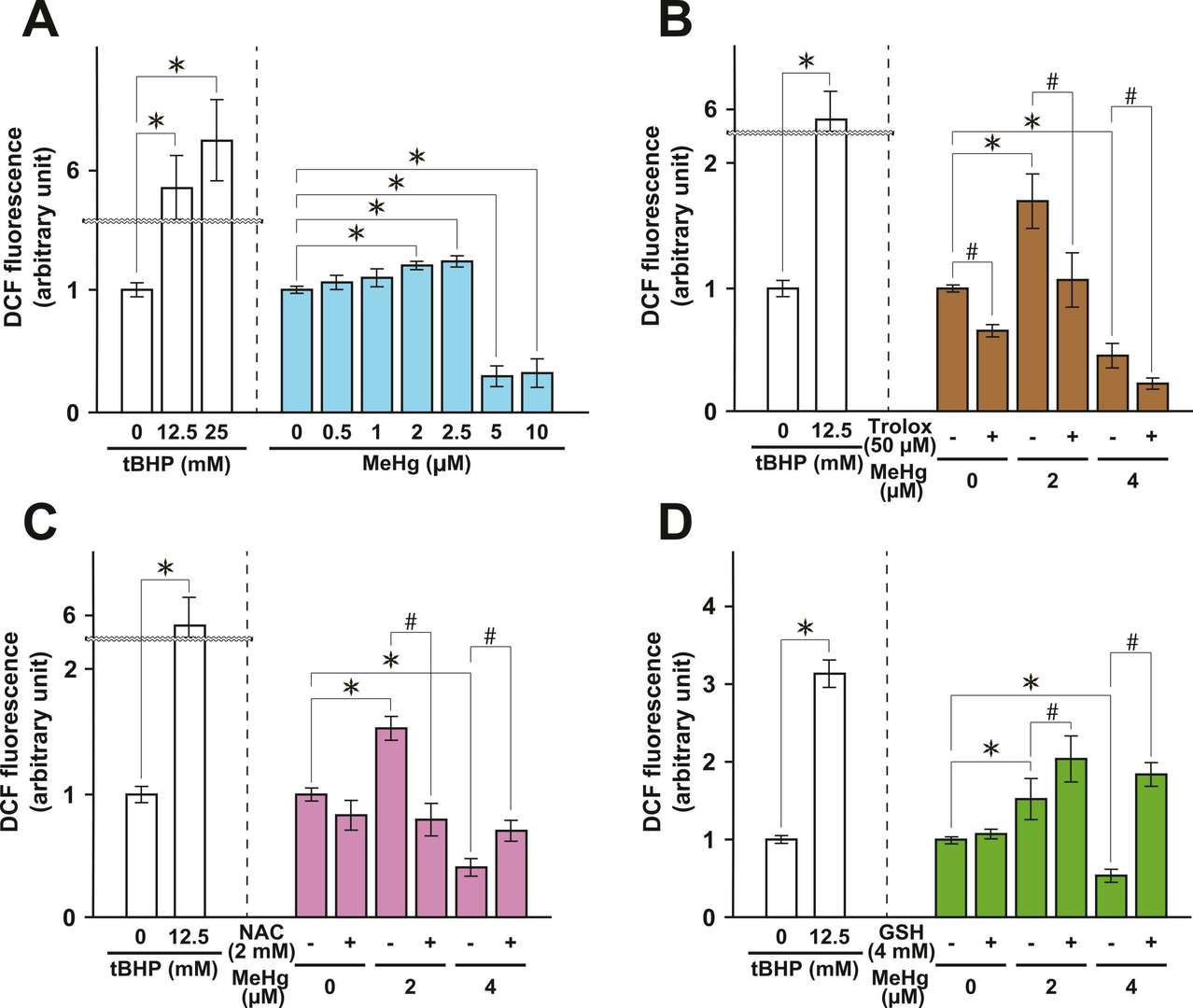 Fig. 2. Dose-dependent alterations in the intracellular ROS level in NRA exposed to MeHg (Sasaki S, Negishi T, et al., 2023).
Fig. 2. Dose-dependent alterations in the intracellular ROS level in NRA exposed to MeHg (Sasaki S, Negishi T, et al., 2023).
Stimulation with BzATP Modified the Cell Size and Migration of Rat Cerebellar Astrocytes In Vitro
The P2X7 receptor, a unique structural and pharmacological member of the nucleotide receptors, plays pivotal roles in cellular communication, especially in the central nervous system. Despite known interactions with immune responses and neurotransmission, its effect on astrocyte morphology and mechanics remains under-explored. Gil-Redondo's team elucidated the P2X7 receptor's influence on the on the morphology, migratory capabilities, and mechanical properties of rat cerebellar astrocytes using selective agonists BzATP, time-lapse video microscopy and atomic force microscopy (AFM) techniques.
Cerebellar astrocytes were treated with or without P2X7R agonist BzATP (300 µM) for 1 hour and monitored using time-lapse microscopy for 3 hours to examined whether P2X7 receptor activation affects cell size and migration. BzATP treatment increased astrocyte size significantly (1001 µm² vs. 812 µm² for controls) as shown in Fig. 3, effects blocked by P2X7R antagonist A43 (765 µm²). BzATP also increased membrane protrusions, reduced by A43 (Fig. 3a and c). They examined cell migration by measuring how far astrocytes moved. BzATP significantly increased this distance, an effect also blocked by A43, confirming P2X7R's role (Fig. 4). Table 1 provides a summary of the effects of different treatments on cell size, protrusions, and migration, highlighting the reversal of BzATP effects by A43. Overall, P2X7R stimulation causes cells to change in shape and movement, suggesting the mechanical properties of astrocytes adjust during this process. Visual analysis alone cannot quantify these changes, indicating the need for additional techniques.
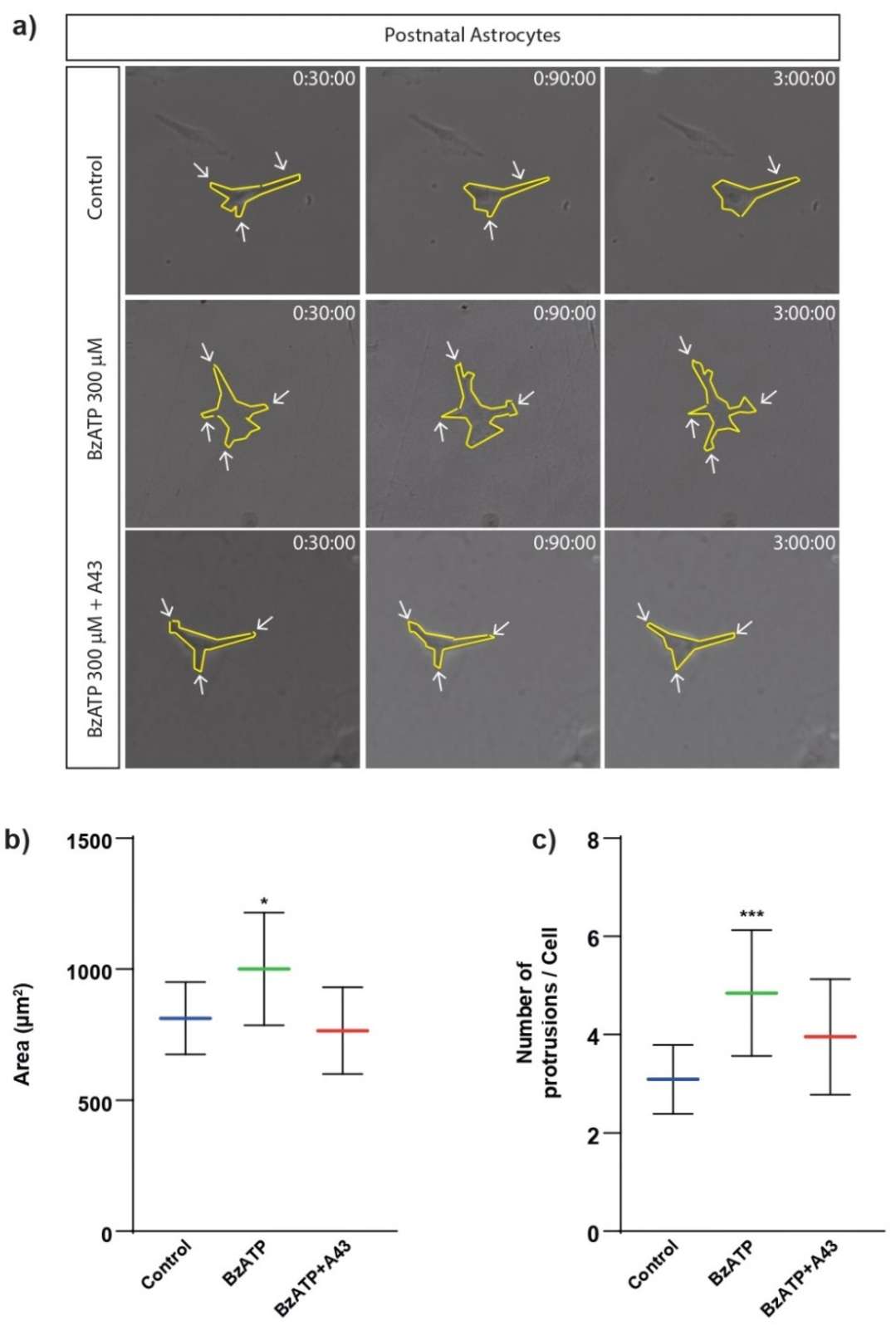 Fig. 3. Effects of BzATP treatment on cell size and membrane protrusions of rat cerebellar astrocytes (Gil-Redondo JC, Iturri J, et al., 2022).
Fig. 3. Effects of BzATP treatment on cell size and membrane protrusions of rat cerebellar astrocytes (Gil-Redondo JC, Iturri J, et al., 2022).
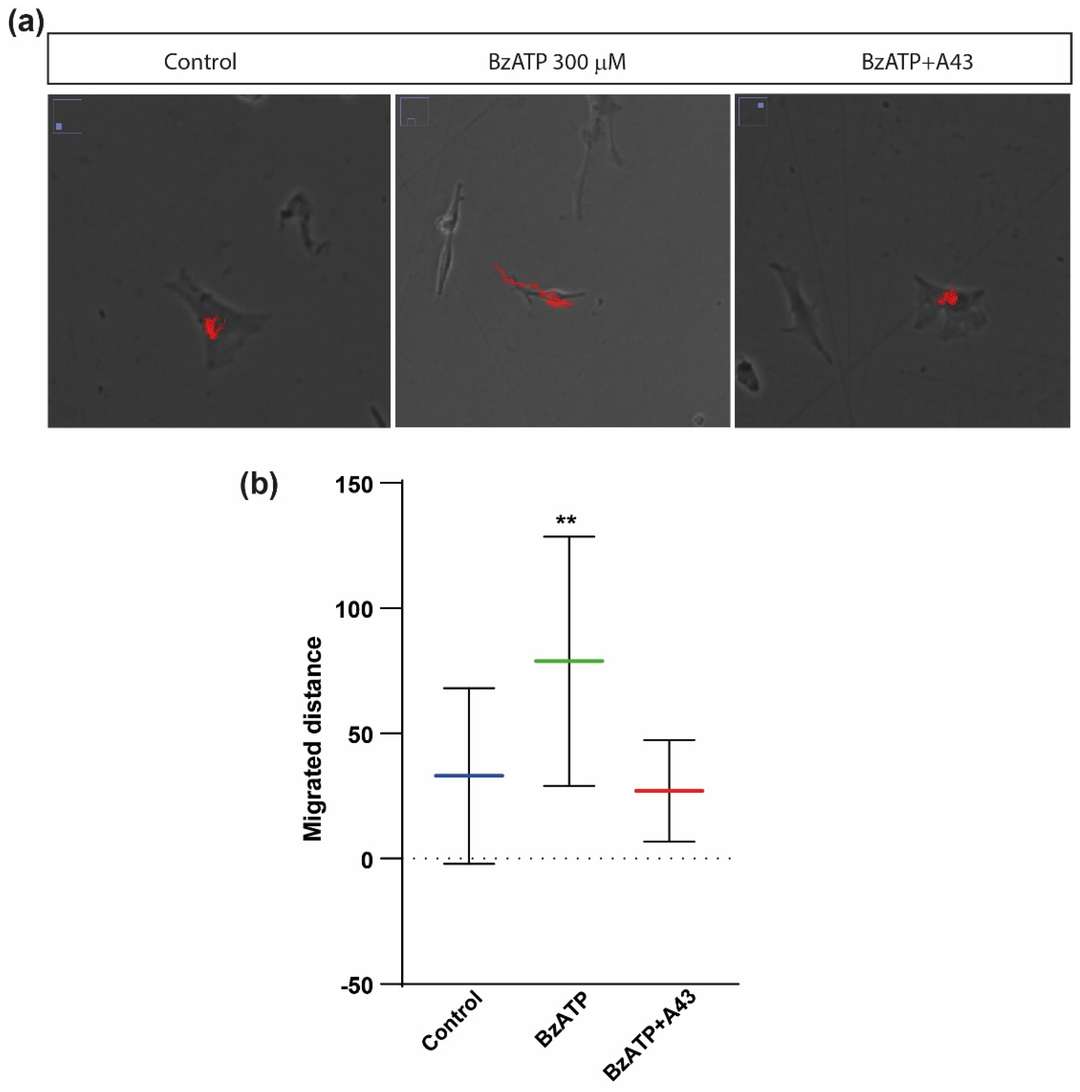 Fig. 4. Effects of BzATP treatment on migratory capacity of rat cerebellar astrocytes (Gil-Redondo JC, Iturri J, et al., 2022).
Fig. 4. Effects of BzATP treatment on migratory capacity of rat cerebellar astrocytes (Gil-Redondo JC, Iturri J, et al., 2022).
Ask a Question
Write your own review