ONLINE INQUIRY
Mouse Renal Glomerular Endothelial Cells
Cat.No.: CSC-C9371W
Species: Mouse
Source: Kidney
Morphology: Polygonal
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Mouse renal glomerular endothelial cells (MRGECs) are specialized microvascular endothelial cells that are found in the glomerular capillaries, and that inner membrane is the glomerular filtration barrier. These cells have a flat shape, slightly flattened nuclei, and their bodies reach into the capillary wall. Notably, MRGECs cells express endothelial cell markers like CD31, CD105, VE-cadherin, GSL I-B4 and ULEX, which help them be identified and purified in experiments. GECs are characterized by their densely fenestrated form, with efficient transport of the solutes and fluids along the glomerular basement membrane. As the primary filter of the glomerular filtration membrane, MRGECs limit capillary permeability and precisely regulate the filtration rate of water, electrolytes and sludge for fluid and electrolyte distribution in the body. Moreover, MRGECs in glomerular inflammation can control immune cell migration and adherence to actively participate in inflammatory response onset and evolution. They also release inflammatory mediators and cytokines. Furthermore, MRGECs produce and release a range of bioactive molecules including nitric oxide and endothelin to control the tone of arteries and the flow of glomeruli. And they release extracellular matrix elements, too, helping to heal and regenerate the glomerular basement membrane.
These features make MRGECs useful in generating in vitro models of glomerular disorders such as glomerulonephritis and diabetic nephropathy. Such models make it easier to understand the mechanisms of disease, pathophysiology and what might be targeted for therapy. In addition, as MRGECs are endothelial, they can be used to research basic vascular biology topics such as angiogenesis, endothelial cell signaling and cell-cell interactions.
 Fig. 1. CD31 staining of glomerular endothelial cells on light microscopy (Takano K, Kawasaki Y, et al., 2007).
Fig. 1. CD31 staining of glomerular endothelial cells on light microscopy (Takano K, Kawasaki Y, et al., 2007).
Role of Ferroptosis in LPS-Induced AKI under In Vitro Conditions
Sepsis-associated acute kidney injury (SA-AKI) results in significant morbidity and mortality, and ferroptosis may play a role in its pathogenesis. Sepsis leads to the overproduction of reactive oxygen species (ROS), causing cell damage and organ dysfunction, notably affecting the kidneys.
Zhang's team utilized male C57BL/6 mice induced with sepsis through cecal ligation and puncture (CLP) and examines the effects of GYY4137 on ferroptosis and AKI. Results showed that BUN and Cre levels and the degree of ferroptosis were highest at 24 h in the above experiment. Then, they tested the role of GYY4137 in ferroptosis modulation in in vitro assessments using mouse renal glomerular endothelial cells exposed to LPS. A CCK-8 assay revealed that 100 µg/mL LPS treatment significantly reduced MRGECs viability at 24 hours, likely due to ferroptosis (Fig. 1A). However, this reduced viability improved with the application of the ferroptosis inhibitor Fer-1 at 10 µM (Fig. 1B). Analysis of ferroptosis-related protein expression in MRGECs treated with varying LPS concentrations (0, 0.1, 1, 10, and 100 µg/mL) for 24 hours confirmed intracellular ferroptosis level increased with LPS concentration, peaking at 100 µg/mL (Fig. 1C–F). Fer-1 effectively countered ferroptosis in cells treated with 100 µg/mL LPS by increasing Gpx4 protein levels and decreasing TF and FTH-1 protein levels, compared to cells not treated with Fer-1 (Fig. 1G–J).
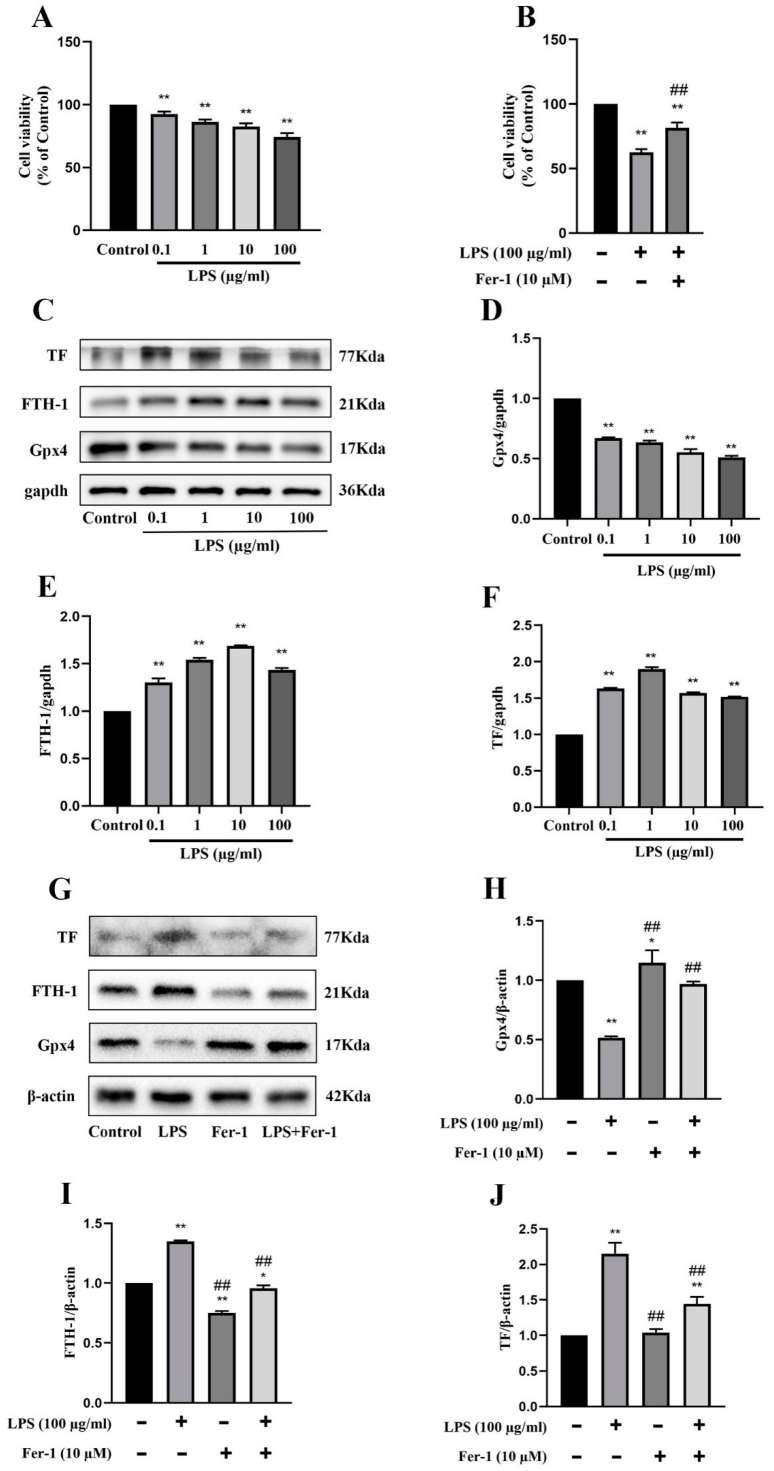 Fig. 1. Exacerbation of ferroptosis and AKI in LPS-treated MRGECs (Zhang L, Rao J, et al., 2023).
Fig. 1. Exacerbation of ferroptosis and AKI in LPS-treated MRGECs (Zhang L, Rao J, et al., 2023).
Allograft Inflammatory Factor-1 Enhances Inflammation and Oxidative Stress via the NF-κB Pathway in Diabetic Kidney Disease
DKD, the primary cause of end-stage renal disease worldwide, is associated with diabetic prevalence and characterized by endothelial cell dysfunction and inflammation. AIF-1, a protein known for its role in inflammation, may contribute to endothelial dysfunction in DKD through the NF-κB pathway. Fu's team hypothesized that AIF-1 promotes glomerular endothelial cell inflammation and oxidative stress via the NF-κB pathway in DKD, and tested their hypothesis in vivo and in vitro.
Mouse renal glomerular endothelial cells (MRGECs) were cultured for in vitro assessments. AIF-1 was overexpressed using lentivirus, confirmed by Q-PCR (Fig. 2B). ELISA results revealed that high glucose (30 mM) significantly increased IL-6, TNF-α, and ROS levels, further elevated in AIF-1 overexpressing cells (Fig. 2C-E). Conversely, T-SOD expression decreased (Fig. 2F). These findings indicate AIF-1 enhances oxidative stress and inflammation under high glucose in MRGECs. Western blot and immunofluorescence showed high glucose induced IκBa phosphorylation and NF-κBp65 nuclear translocation, both enhanced by AIF-1 overexpression (Fig. 2G-I). Overall, AIF-1 enhances NF-κB pathway activity at high glucose levels in MRGECs. Western blot showed that NF-κB inhibitor BAY 11–7082 reduced p-IkBa and nuclear NF-κBp65 levels (Fig. 3A and B). ELISA results demonstrated that BAY 11–7082 reversed the elevated IL-6 and TNF-α levels caused by AIF-1 overexpression (Fig. 3C and D). ROS levels followed a similar pattern (Fig. 3E), while T-SOD levels were inversely related (Fig. 3F). These findings indicate that NF-κB activation enhances AIF-1-driven inflammation in MRGECs.
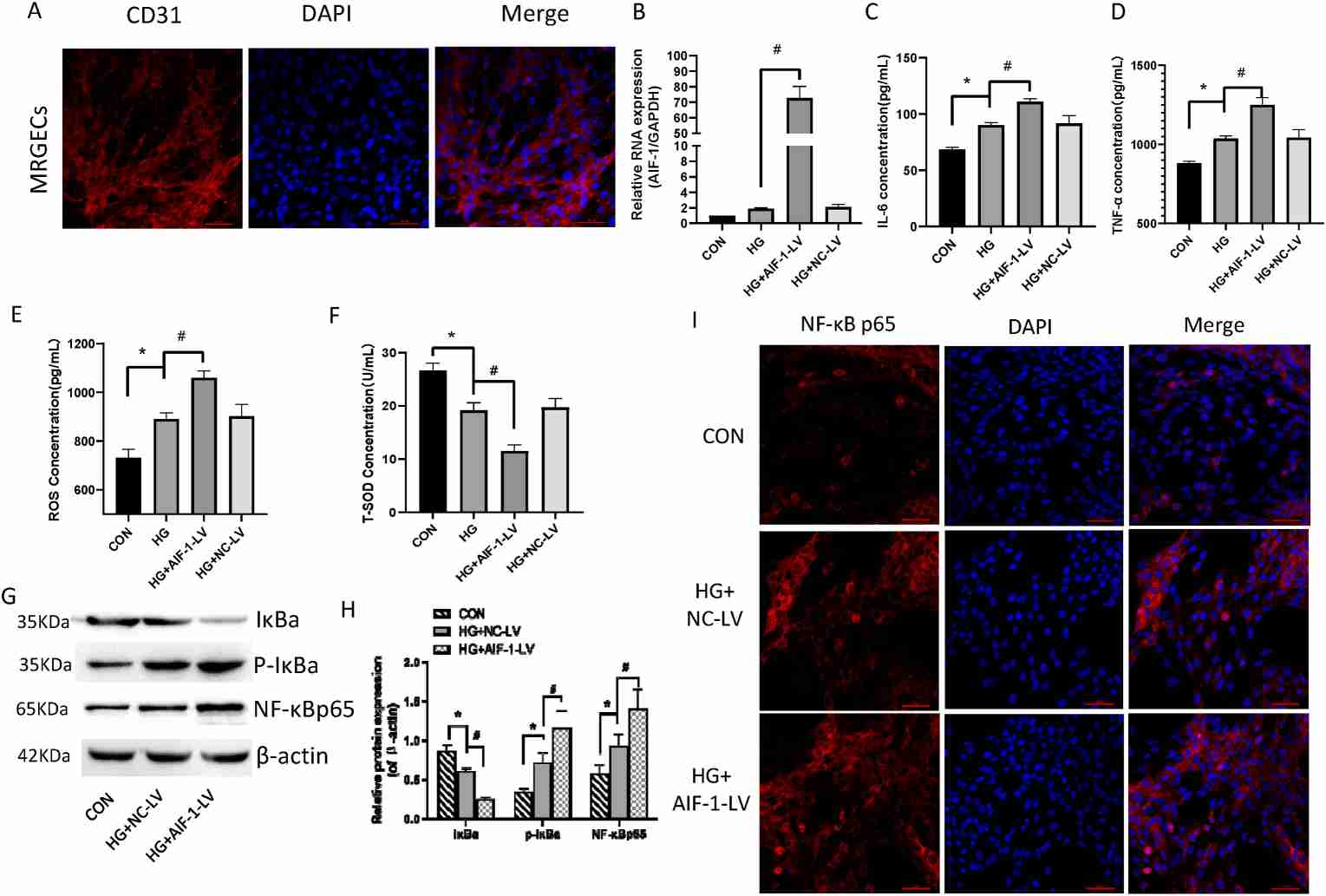 Fig. 2. AIF-1 elevated high glucose-induced inflammation and oxidative stress and activated the NF-κB pathway in MRGECs (Fu Y, Wang X, et al., 2022).
Fig. 2. AIF-1 elevated high glucose-induced inflammation and oxidative stress and activated the NF-κB pathway in MRGECs (Fu Y, Wang X, et al., 2022).
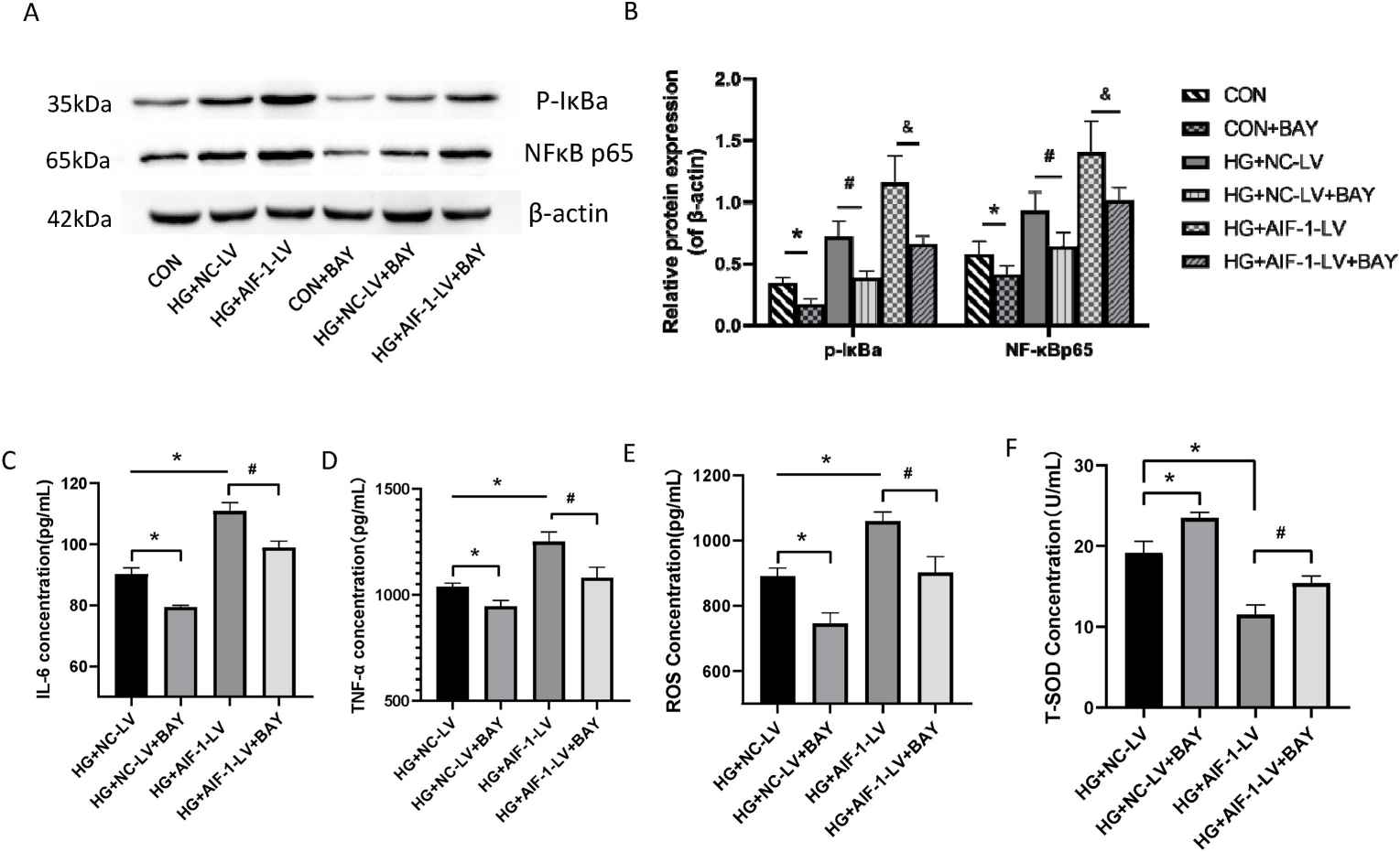 Fig. 3. NF-κB pathway activation contributed to AIF-1-induced inflammation in MRGECs (Fu Y, Wang X, et al., 2022).
Fig. 3. NF-κB pathway activation contributed to AIF-1-induced inflammation in MRGECs (Fu Y, Wang X, et al., 2022).
Ask a Question
Write your own review