ONLINE INQUIRY

Mouse Astrocytes Cortex
Cat.No.: CSC-C4465X
Species: Mouse
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The mouse astrocyte cortex cell line is primarily derived from mouse brain cortical tissue. During mouse development, radial glial cells (RGCs) lose their neurogenic potential and gradually acquire most of the characteristics of astrocytes. As development progresses, most astrocyte precursors are generated, which then migrate to the white or gray matter, differentiating into fibrous and protoplasmic astrocytes, respectively. Mosaic analysis with double markers (MADM) and clonal analysis indicate that 1/6 of the neurogenic radial glial cortical progenitors produce neuroglial cells, while the remaining RGCs eventually directly differentiate into astrocytes. Throughout the differentiation process, these cells undergo morphological changes, losing apical contact, becoming unipolar, retracting radial fibers, and then relocating their cell bodies away from the ventricular zone to become multipolar, thus acquiring their astrocytic morphology. They perform various functions, including supporting and maintaining neuronal and synaptic function, forming connections with over 100,000 synapses, which likely reflects the processing of numerous synaptic activities involved in handling sensory information. Furthermore, astrocytes play crucial roles in neuronal survival and synaptic function, including neurotransmitter recycling and potassium ion buffering. They also actively participate in synapse formation and pruning.
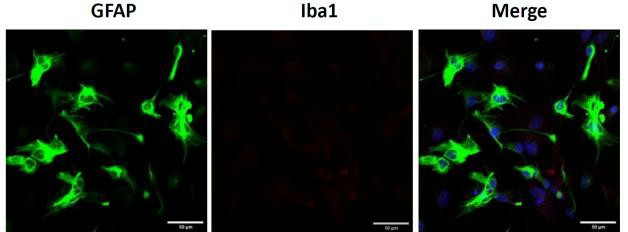 Fig. 1. Primary cortical astrocytes. Representative image of GFAP and Iba1 immunostaining of cortical astrocytes (Sun Y, Winters A, et al., 2023).
Fig. 1. Primary cortical astrocytes. Representative image of GFAP and Iba1 immunostaining of cortical astrocytes (Sun Y, Winters A, et al., 2023).
Overexpression of Catalytically Active, but not Mutated/Inactive, 17ΒHSD10 Inhibits Mitochondrial Respiration in Cortical Astrocytes
Alzheimer's disease (AD) and post-ischemic neurodegeneration share mechanisms like brain metabolic dysfunction and amyloid accumulation. Astrocytes, key to these processes, have an understudied mitochondrial role. Metodieva's team used in vitro murine astrocyte culture models to explore the enzymatic activity of 17β-hydroxysteroid dehydrogenase type 10 (17βHSD10) in astrocytes under metabolic and amyloidogenic stress.
Metodieva's team used a lentiviral system to overexpress wild-type (wt) and mutant (mut) 17βHSD10 in astrocyte mitochondria. They examined how it affects mitochondrial respiration in cortical astrocytes (Fig. 1). Mitochondrial stress testing (Fig. 1A) showed that wt-17βHSD10 overexpression did not impact basal respiration but reduced maximal respiratory capacity by 45% and spare capacity by 65% (Fig. 1D). While both control and mut-17βHSD10 cells increased their respiration by nearly 70%, the increase in wt-17βHSD10 cells was significantly lower at 30% above baseline (Fig. 1E). To verify if excess enzymatic activity of wt-17βHSD10 caused respiratory changes, they used a potent inhibitor, AG18051. Pretreatment with AG18051 restored all respiratory parameters to control levels in astrocytes overexpressing wt-17βHSD10 (Fig. 1C–E), confirming the role of excess activity in respiratory inhibition. It also enhanced respiration and capacity in astrocytes with normal 17βHSD10 levels (Extended Data Fig. 1-1). Mitochondrial decoupling (FCCP) didn't affect 17βHSD10 activity, but ETC inhibition (Complexes I, III, V) reduced it (Fig. 1F). Thus, while 17βHSD10 activity relies on ETC, excess activity may limit astrocytes' respiratory adaptability to energy demands.
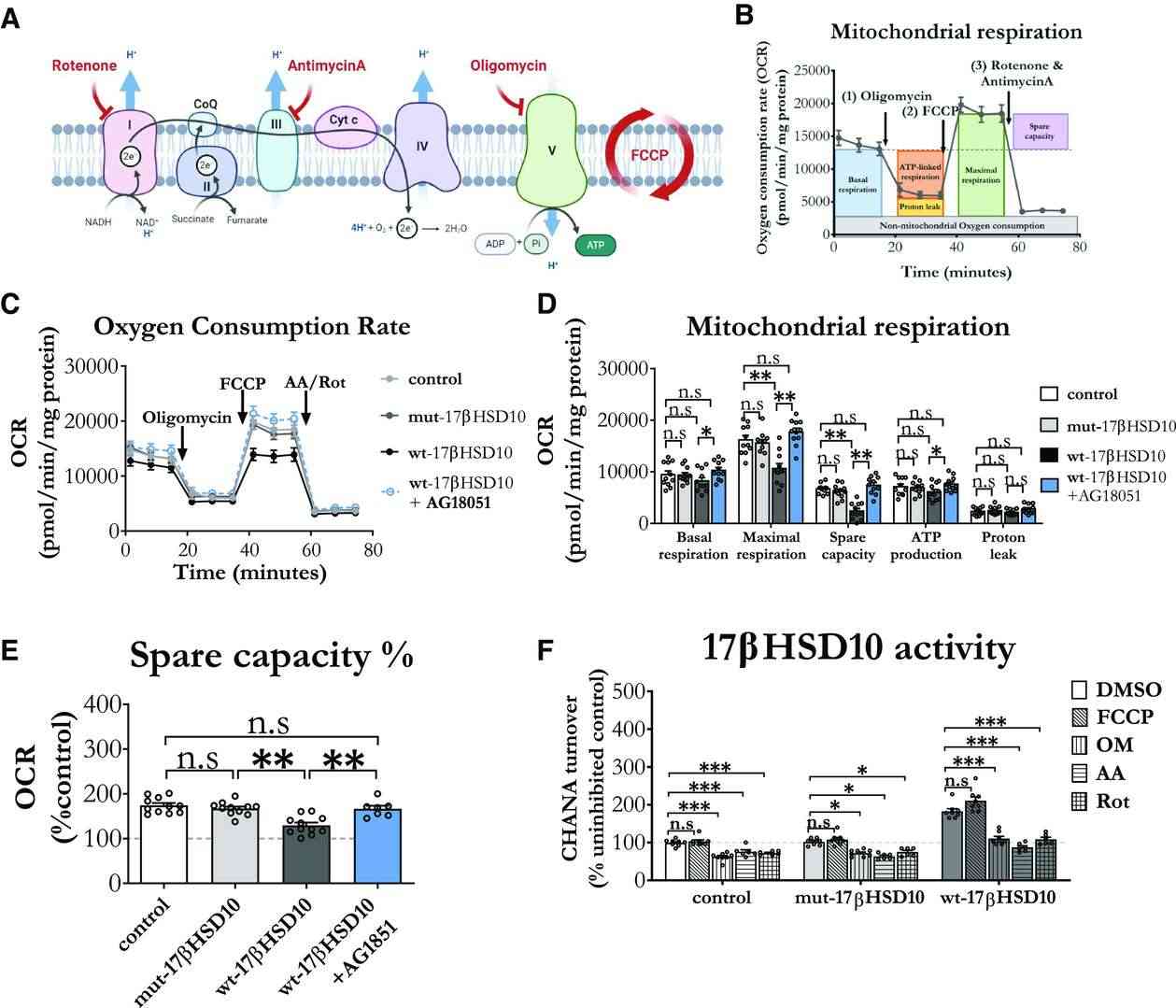 Fig. 1. Increased 17βHSD10 activity decreased mitochondrial respiration, while ETC inhibition decreased the activity of the enzyme and AG18051 countered these effects (Metodieva V, Smith T, et al., 2022).
Fig. 1. Increased 17βHSD10 activity decreased mitochondrial respiration, while ETC inhibition decreased the activity of the enzyme and AG18051 countered these effects (Metodieva V, Smith T, et al., 2022).
OxyR Treatment Activates Autophagic Flux in Mouse Cortical Astrocytes and Rat Cortical Neurons
Astrocyte accumulation is one of the earliest neuropathological changes in Alzheimer's disease (AD), and amyloid precursor protein (APP) is the hallmark of AD. Oxyresveratrol (OxyR), a potent antioxidant derived from the mulberry plant, has demonstrated neuroprotective effects by modulating amyloid precursor protein (APP) through autophagy pathways. Its ability to cross the blood-brain barrier makes it a promising candidate for neurodegenerative disorder treatments.
Rahman et al. used primary cortical astrocytes to investigate OxyR's effect on the AMPK/ULK1/mTOR-dependent autophagy pathway. To analyze OxyR-induced autophagic flux, they utilized chloroquine (CQ), a late-stage autophagy inhibitor, and assessed autophagic activities using immunostaining and immunoblotting. CQ blocks lysosome-autophagosome fusion. OxyR treatment increased LC3 puncta, while CQ prevented autophagosome-lysosome binding, leading to more LC3 puncta in astrocytes (Fig. 2A and B). Immunoblotting showed that OxyR and CQ together elevated LC3-II expression in astrocytes (Fig. 2C and D), indicating enhanced autophagy. In rat neurons, co-treatment resulted in more LC3 puncta and LC3-II expression (Fig. 2E–H). OxyR reduced p62 in both cell types, and CQ-induced p62 increase confirmed autophagic flux stimulation by OxyR.
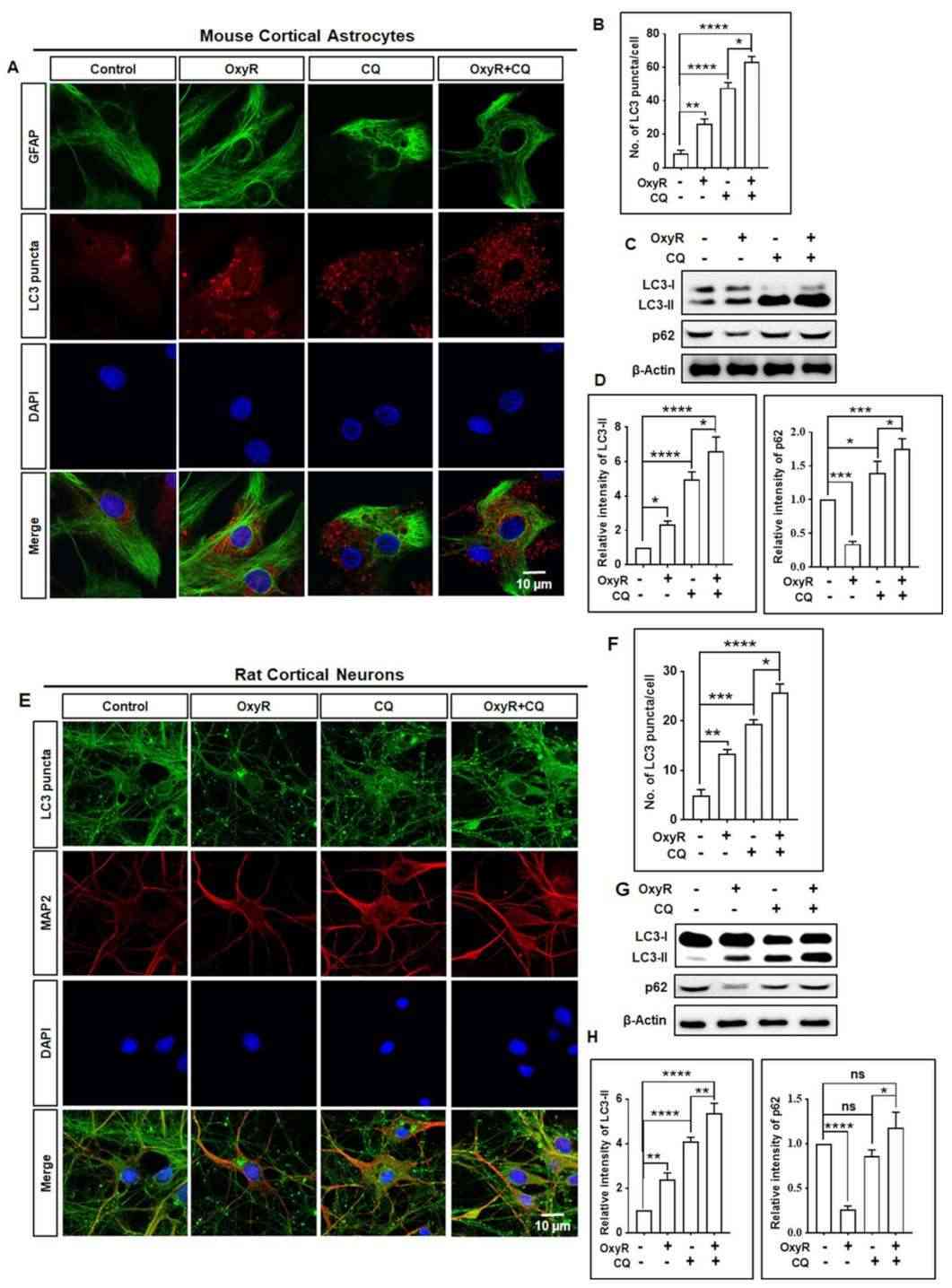 Fig. 2. OxyR treatment induced autophagic flux in cortical astrocytes and neurons (Rahman MA, Cho Y, et al., 2021).
Fig. 2. OxyR treatment induced autophagic flux in cortical astrocytes and neurons (Rahman MA, Cho Y, et al., 2021).
Ask a Question
Write your own review