ONLINE INQUIRY
Mouse Astrocytes Cerebellar
Cat.No.: CSC-C4466X
Species: Mouse
Source: Brain
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse Astrocytes Cerebellar (MA-c) cell line was isolated from mouse cerebellum and is usually isolated from juvenile animals. These cells fold around cerebellar neurons and fill the spaces between them, forming with neurons and blood vessels the layered structure of the cerebellum. Astrocytes occupy the cerebellar cortex and deep nuclei and are involved in keeping the cerebellum intact and functioning. The MA-c cells are efficient in transporting glucose and amino acids from the bloodstream or the interstitial fluid to neurons, providing energy for neurons and supporting their metabolism and function. Also, MA-c cells balance extracellular ions (primarily potassium ions) via ion channels and transporters on their cell membranes. Potassium ions released during neuronal excitability are rapidly digested by astrocytes to prevent toxic buildup and restore normal neuronal excitability and electrophysiology. MA-c cells also contain neurotransmitter transporters and associated enzymes, which help them systematically flush out excess neurotransmitters like glutamate from synaptic clefts to terminate signaling and facilitate metabolism. Many cerebellar disorders, including ataxia and cerebellar atrophy, have functional astrocyte abnormalities. In this way, MA-c cell lines can be used to build in vitro disease models to investigate the causes of these disorders.
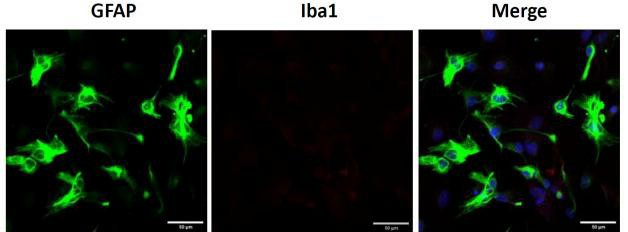 Fig. 1. Primary cerebellar astrocytes. Representative image of GFAP (left) and Iba1 (middle) immunostaining of cerebellar astrocytes, scale bar = 20 μm (Sun Y, Winters A, et al., 2023).
Fig. 1. Primary cerebellar astrocytes. Representative image of GFAP (left) and Iba1 (middle) immunostaining of cerebellar astrocytes, scale bar = 20 μm (Sun Y, Winters A, et al., 2023).
S-equol Increased the Proliferation, Invasion, Lamellipodia Formation, and Rearrangement of Cortical F-actin Activity in Mouse Cerebellar Astrocytes
Soy isoflavone metabolite S-equol is a phytoestrogen receptor binder for estrogen receptors, which influences many brain areas including the cerebellum. Although widely known, it has not been fully investigated for its impact on cerebellar development and function. Ariyani's team used mouse primary cerebellar cultures, Neuro-2A cells and astrocyte-enriched cultures to explore how S-equol affected cerebellar development by targeting estrogen receptors and G-protein coupled receptors in neurons and astrocytes.
Studies in the primary cerebellar culture demonstrated that S-equol increased the dendrite arborisation of purkinje cells and the neurite outgrowth of neuro-2A cells. To investigate whether S-equol promoted astrocyte proliferation, they performed a BrdU incorporation assay, MTS cell proliferation assay, and immunocytochemistry for pERK1/2. S-equol dose-dependently increased proliferation (1-100 nM) in a BrdU assay (Fig. 1A). An MTS assay showed that S-equol enhanced astrocyte proliferation at 24, 48, and 72 hours. Proliferation was inhibited by G15 (10 nM), but ICI (10 nM) had a minor effect only after 72 hours (Fig. 1B). S-equol increased pERK1/2 positive cells within 30 minutes, and this was inhibited by G15 (Fig. 3C), indicating proliferation via the GPR30 pathway. For S-equol-induced astrocyte migration, invasion assays and F-actin immunocytochemistry were performed. S-equol (10 nM) boosted astrocyte invasion, inhibited by G15 (Fig. 2A). Stress fiber formation and the cortical F-actin score (CFS) increased with S-equol and were suppressed by G15, not ICI (Fig. 2B). This suggests GPR30's key role in astrocyte migration.
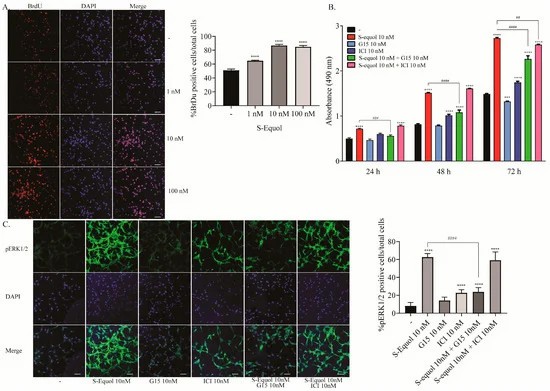 Fig. 1. Effects of S-equol on mouse cerebellar astrocyte proliferation (Ariyani W, Miyazaki W, et al., 2019).
Fig. 1. Effects of S-equol on mouse cerebellar astrocyte proliferation (Ariyani W, Miyazaki W, et al., 2019).
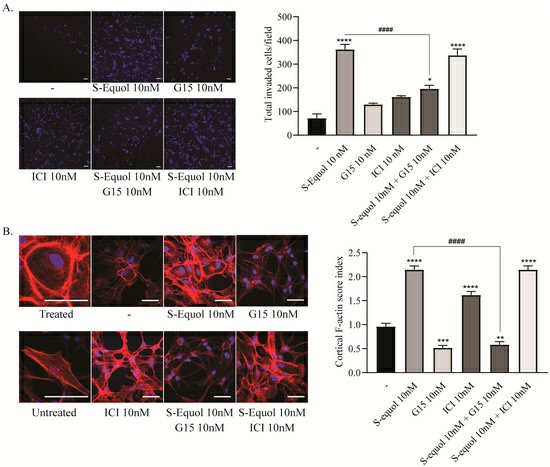 Fig. 2. Effects of S-equol on mouse cerebellar astrocyte invasion and F-actin rearrangement (Ariyani W, Miyazaki W, et al., 2019).
Fig. 2. Effects of S-equol on mouse cerebellar astrocyte invasion and F-actin rearrangement (Ariyani W, Miyazaki W, et al., 2019).
Neuron-glia Interaction Enhanced TH-induced Neuritogenesis
Membrane-associated receptors are vital in brain development, with thyroid hormones (TH) affecting both nuclear and membrane-associated receptors, particularly integrin αvβ3 in neurons and glia. Integrin αvβ3 influences cellular events like morphogenesis and synaptogenesis. However, its role in brain development remains unclear.
Ariyani et al. investigated TH's effects via integrin αvβ3 on neurons and astrocytes using primary cerebellar culture, astrocyte-enriched culture, Neuro-2A clonal cells, and co-culture of neurons and astrocytes. The study confirmed that TH promotes dendrite growth, which is hindered by inhibiting integrin αvβ3 or TRα. Additionally, TH impacts protein phosphorylation through TRα-dependent pathways, vital for neuritogenesis, indicating a role for neuron-astrocyte interaction. To examine the effects of TH exposure on neuron-glia interactions, they performed a co-culture study. Neuro-2A cells cultured with cerebellar astrocytes enriched culture for 3-5 days were stained with β-tubulin III and anti-doublecortin (DCX). In co-cultures, DCX stained Neuro-2A and astrocyte soma, so β-tubulin III and F-actin were used to study cell interactions. Photographs of Neuro-2A and co-cultures are shown (Fig. 3A). Neurite growth was measured using Sholl analysis, showing enhanced TH-induced neurite branching and elongation in co-cultures (Fig. 3B-D). Inhibiting integrin αvβ3 reduced these effects (Fig. 4), indicating its importance in neuron-glia interaction for neuritogenesis.
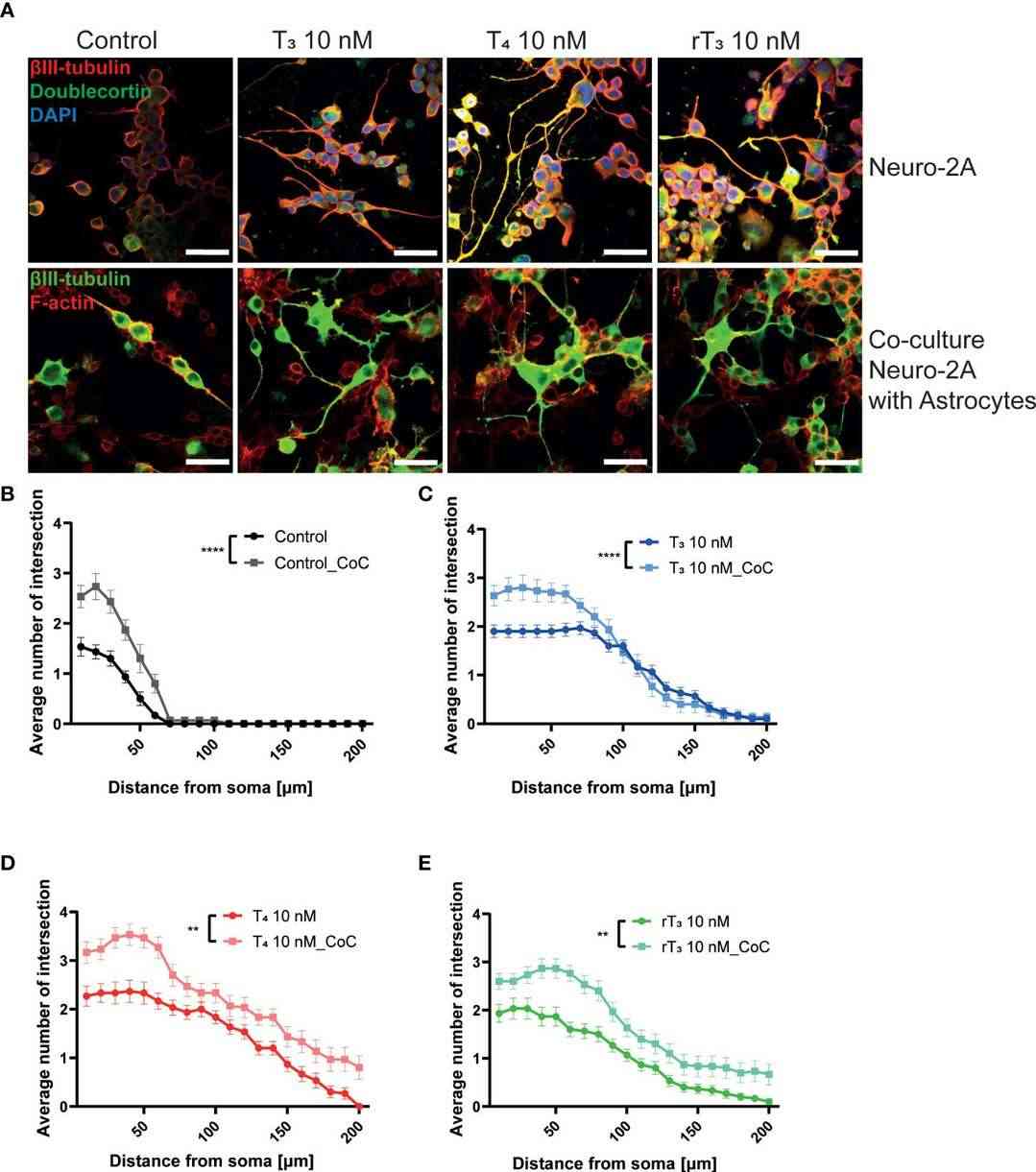 Fig. 3. Co-culture with astrocytes enhanced TH-augmented neurite length in Neuro-2A cells (Ariyani W, Miyazaki W, et al., 2022).
Fig. 3. Co-culture with astrocytes enhanced TH-augmented neurite length in Neuro-2A cells (Ariyani W, Miyazaki W, et al., 2022).
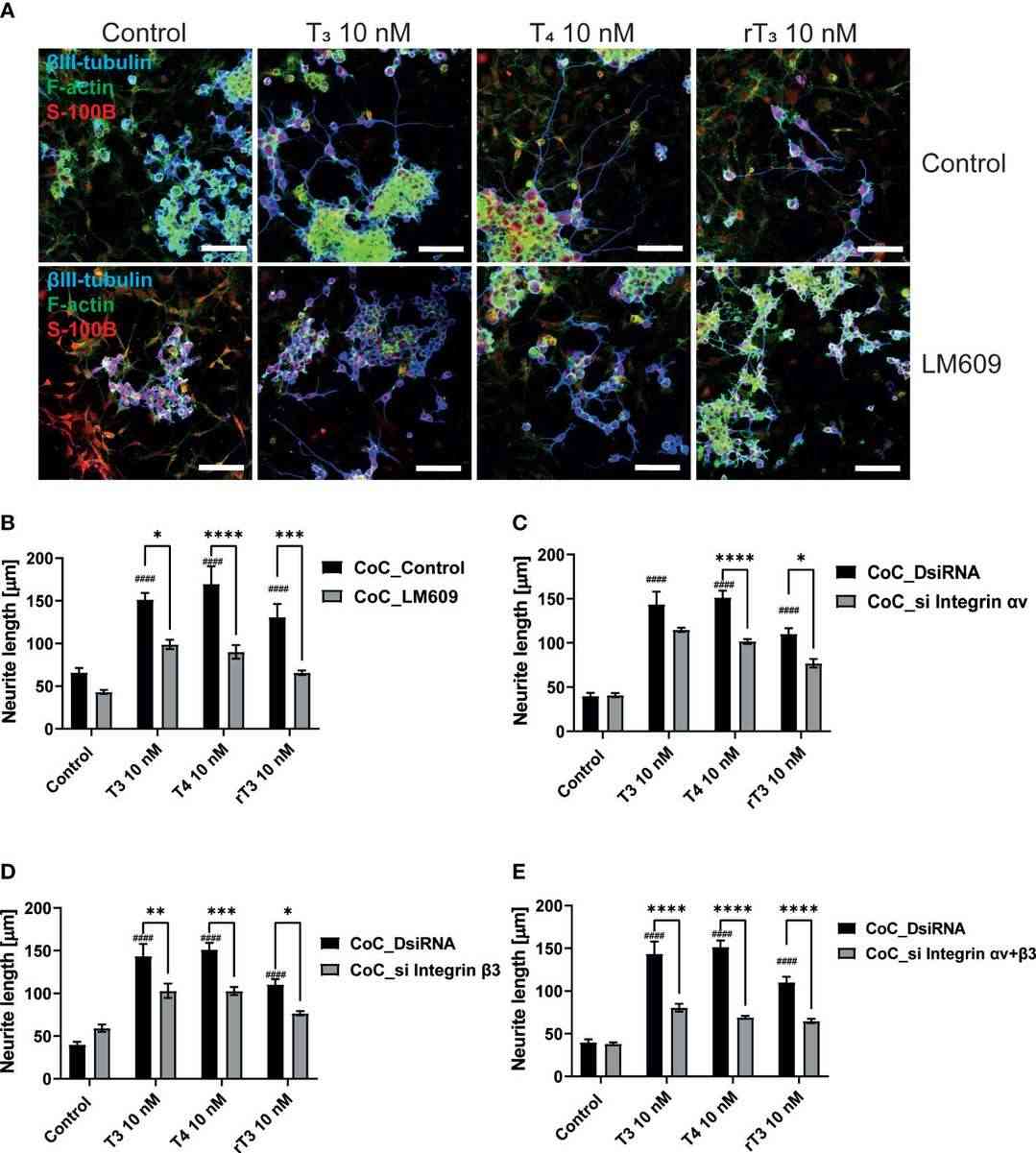 Fig. 4. Integrin αvβ3 affect neurite outgrowth induced by TH in co-culture of astrocytes and Neuro-2A cells (Ariyani W, Miyazaki W, et al., 2022).
Fig. 4. Integrin αvβ3 affect neurite outgrowth induced by TH in co-culture of astrocytes and Neuro-2A cells (Ariyani W, Miyazaki W, et al., 2022).
Ask a Question
Write your own review