ONLINE INQUIRY

Human Renal Epithelial Cells
Cat.No.: CSC-C1560
Species: Human
Source: Kidney
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
HREpiC are isolated from human kidneys. HREpiC are cryopreserved at passage one and delivered frozen. Each vial contains >5 x 10^5 cells in 1 ml volume. HREpiC are characterized by immunofluorescent method with antibodies to cytokeratin-18, -19 and vimentin. HREpiC are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HREpiC are guaranteed to further expand for 15 population doublings in the condition provided by Creative Bioarray.
Human renal epithelial cells, essential to kidney function, constitute the epithelial tissue within the organ. They can be categorized into three primary types based on their location. The first is simple squamous epithelial cells, mostly in the glomerular vascular cavity, separated from mesenchymal cells. They support nephron filtration and balance the glomerular microenvironment. This second type is renal cuboidal epithelial cells or renal tubular epithelial cells. As they are distributed in the renal tubule interstitium, these cells are divided into proximal tubular cells, distal tubular cells and thin segment epithelial cells depending on where they are located in the tubule. And third, simple columnar epithelial cells found primarily on some segments of the collecting ducts like the papillary ducts.
The kidneys' primary function is to filter blood, forming urine to eliminate metabolic waste and excess water. Renal epithelial cells play a critical role in this process. They also reabsorb nutrients such as glucose and amino acids from primary urine and release hydrogen ions to balance acid-base levels. These cells are also immune, and they can initiate inflammation when the kidney is damaged or infected, producing inflammatory mediators and drawing on immune cells for repair and defense.
As they are such an important component of kidney, renal tubular epithelial cells are also a central focus of renal disease research and are often used as in vitro models to mimic the microenvironment of kidneys. These models allow studying cellular modifications of resorption, release and excretion under particular conditions. Sensitive to toxicity, renal tubular epithelial cells are useful in testing potential nephrotoxicity of drugs. Further, they play an important role in renal fibrosis, particularly in stimulating interstitial fibrosis through cytokine release, and their involvement is still under research.
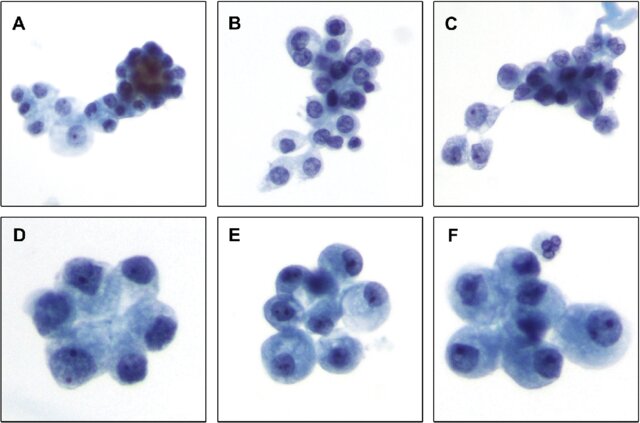 Fig. 1. Typical renal tubular epithelial cells (Bhatnagar, R., Drachenberg, C., et al., 2014).
Fig. 1. Typical renal tubular epithelial cells (Bhatnagar, R., Drachenberg, C., et al., 2014).
Losartan Mitigates Mechanotransduction Properties Alteration and EMT in Renal Epithelial Cells
The biology of kidney cells is constantly under pressure from biomechanical forces. Researchers recently shown that biomechanical stimulus induces EMT by changing the cytoskeleton of kidney epithelial cells. It's long been known that activation of the AT1R leads to pro-fibrotic effects, inflammatory cell recruitment, angiogenesis, cell proliferation and ECM. Meanwhile, angiotensin receptor blockers (ARBs) were shown to inhibit or reverse fibrosis. But there is still no agreement as to the dose-dependence and exact mechanism of ARBs' protective role in renal fibrosis. Huang et al. supposed that the relative lower dose of ARBs could be useful for biomechanical stress-induced renal fibrosis, too.
The most widely used ARB, losartan, albeit at a reduced dose, alleviates renal fibrosis in a unilateral ureteral obstruction (UUO) mouse model. They exposed human renal epithelial cells to a 50 mmHg hydrostatic pressure in vitro to mimic the higher intrarenal hydrostatic pressure of the UUO model. The immunofluorescence staining results showed a significant increase in the total expression of AT1R in renal epithelial cells (Fig. 1A). However, these changes were canceled by adding different concentrations of losartan, one of the most popularly used ARBs. The expression of F-actin displayed dense parallel networks and increased intensity in renal epithelial cells. These changes were almost canceled by losartan also (Fig. 1B). EMT markers were measured at the protein level; in kidney epithelial cells, vimentin, β-catenin, and α-SMA were markedly upregulated while E-cadherin was downregulated (Fig. 2). Predictably, adding 10nM and 100nM losartan to the medium inhibited the hydrostatic pressure-induced alteration of EMT markers in renal epithelial cells (Fig. 2).
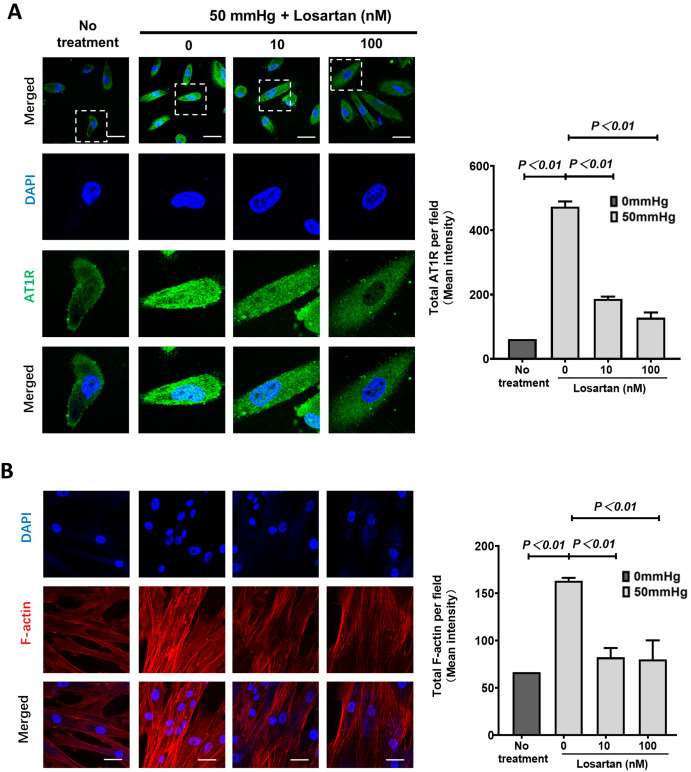 Fig. 1. Immunofluorescence staining on AT1R and F-actin in human renal epithelial cells (Huang Z., Nie H., et al., 2023).
Fig. 1. Immunofluorescence staining on AT1R and F-actin in human renal epithelial cells (Huang Z., Nie H., et al., 2023).
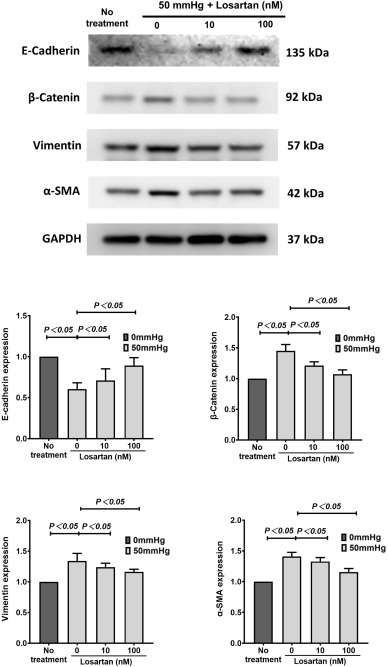 Fig. 2. The expression of E-cadherin, β-catenin, vimentin and α-SMA in human renal epithelial cells (Huang Z., Nie H., et al., 2023).
Fig. 2. The expression of E-cadherin, β-catenin, vimentin and α-SMA in human renal epithelial cells (Huang Z., Nie H., et al., 2023).
Long-term HP Treatment Induced Severe Changes in Cell Morphology and Growth Factor Expression in HREpCs
Obstructive uropathy is a common kidney disease caused by elevated hydrostatic pressure (HP), but the molecular and cellular mechanisms have not been well understood. Chen et al. investigated how elevated HP affects the expression of growth factors in human renal epithelial cells (HREpC), providing new mechanistic insights into kidney injury induced by elevated HP.
After treating HREpC with HP for 48 hours, the cell morphology showed that some HREpC became rounded (Fig. 3A and B). The cell aspect ratio increased but returned to baseline levels within 72 hours after removing HP treatment, indicating that the elongation of HP-treated cells was reversible (Fig. 3C). MTT assay showed a slight decrease in cell viability in HP-treated cells compared to control cells (Fig. 3D). Furthermore, F-actin in HP-treated cells exhibited an elongated morphology (Fig. 3E). The growth factor expression results indicated that the expression of CSF2 and VEGFB was decreased in HP-treated cells after HP removal. The expression of TGFB2 and PDGFB decreased after long-term HP treatment and subsequent removal. Meanwhile, TGFB3 expression increased in HP-treated cells after HP removal. The expression of VEGFA decreased in HP-treated cells 72 hours after HP removal (Fig. 4).
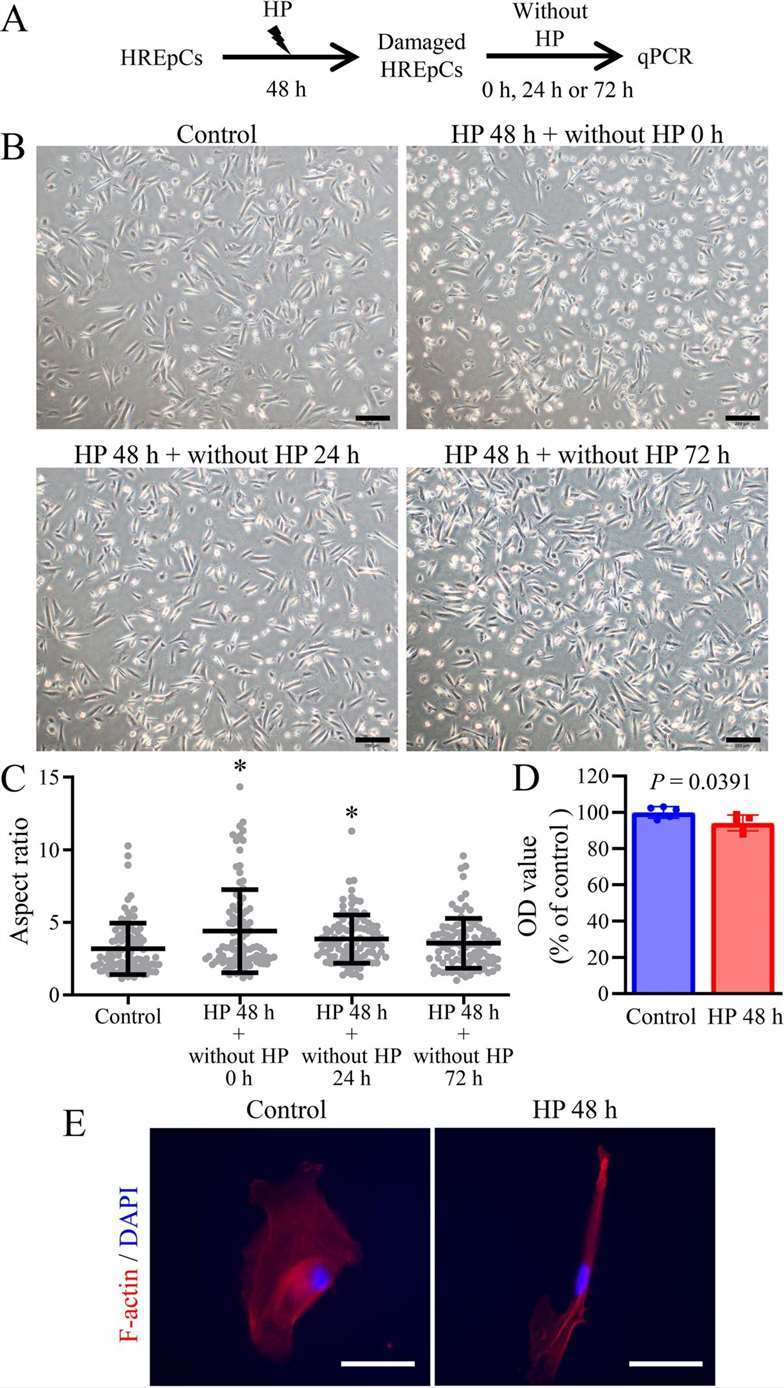 Fig. 3. Long-term HP treatment induced severe changes in cell morphology and growth factor expression in HREpCs (Yan C., Xiao J. et al., 2024).
Fig. 3. Long-term HP treatment induced severe changes in cell morphology and growth factor expression in HREpCs (Yan C., Xiao J. et al., 2024).
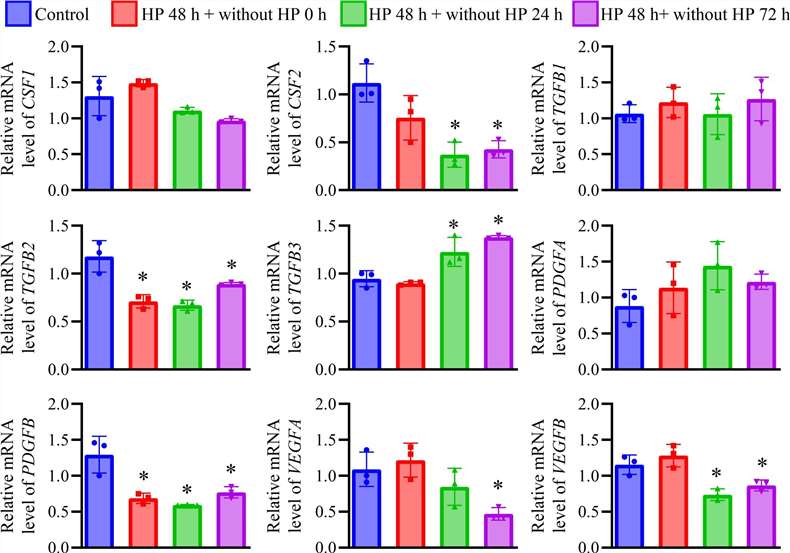 Fig. 4. The expression of growth factors in HREpCs received 100 cmH2O HP treatment for 48 h (Yan C., Xiao J. et al., 2024).
Fig. 4. The expression of growth factors in HREpCs received 100 cmH2O HP treatment for 48 h (Yan C., Xiao J. et al., 2024).
Human Renal Epithelial Cells from Creative Bioarray are comprised of cells from the cortex and glomerular region.
Ask a Question
Write your own review