ONLINE INQUIRY

Human Monocytes (hMo-PB)
Cat.No.: CSC-C1671
Species: Human
Source: Peripheral Blood; Blood
Cell Type: Monocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
In addition, hMo-PB are available which are pre-screened for their differentiation capacity into dendritic cells.
Human peripheral blood monocytes are mononuclear cells isolated from peripheral blood by gradient separation. They account for 10-30% of peripheral blood mononuclear cells, along with lymphocytes (T cells, B cells, and NK cells) and dendritic cells. Monocytes are the largest leukocytes, measuring 12-15 μm in diameter. They are round or oval in shape with folds and pseudopods on their surface. They are derived from hematopoietic stem cells in the bone marrow and, as they differentiate, grow into monocytes before being released into peripheral blood. After staying in the blood for 3-6 days, the monocytes move out of the blood vessels into tissue or body cavities and transform into macrophages within 5-9 days, becoming the mononuclear phagocyte system. Monocytes are also essential to immune response; they can phagocytize and digest antigens, and transmit antigen data to lymphocytes, thus triggering specific immune responses.
The monocyte count is an important indicator in disease diagnosis when take blood tests. Changes in monocyte counts are closely associated with the onset and progression of various diseases. For example, monocyte numbers may fluctuate in infections, inflammation and tumors. Tracking changes in monocyte counts throughout treatment allows assessment of therapeutic performance and survival. Therefore, peripheral blood monocytes are often recruited in disease experiments to test immunity. By culturing, stimulation and monitoring of monocytes in peripheral blood, scientists explore their mechanisms of immunity, immunity control and immunity tolerance. Furthermore, in research and development, the observation of how drugs influence peripheral blood monocyte proliferation, apoptosis and cytotoxicity help to estimate the potential immunologic or therapeutic activities of the drugs before developing them.
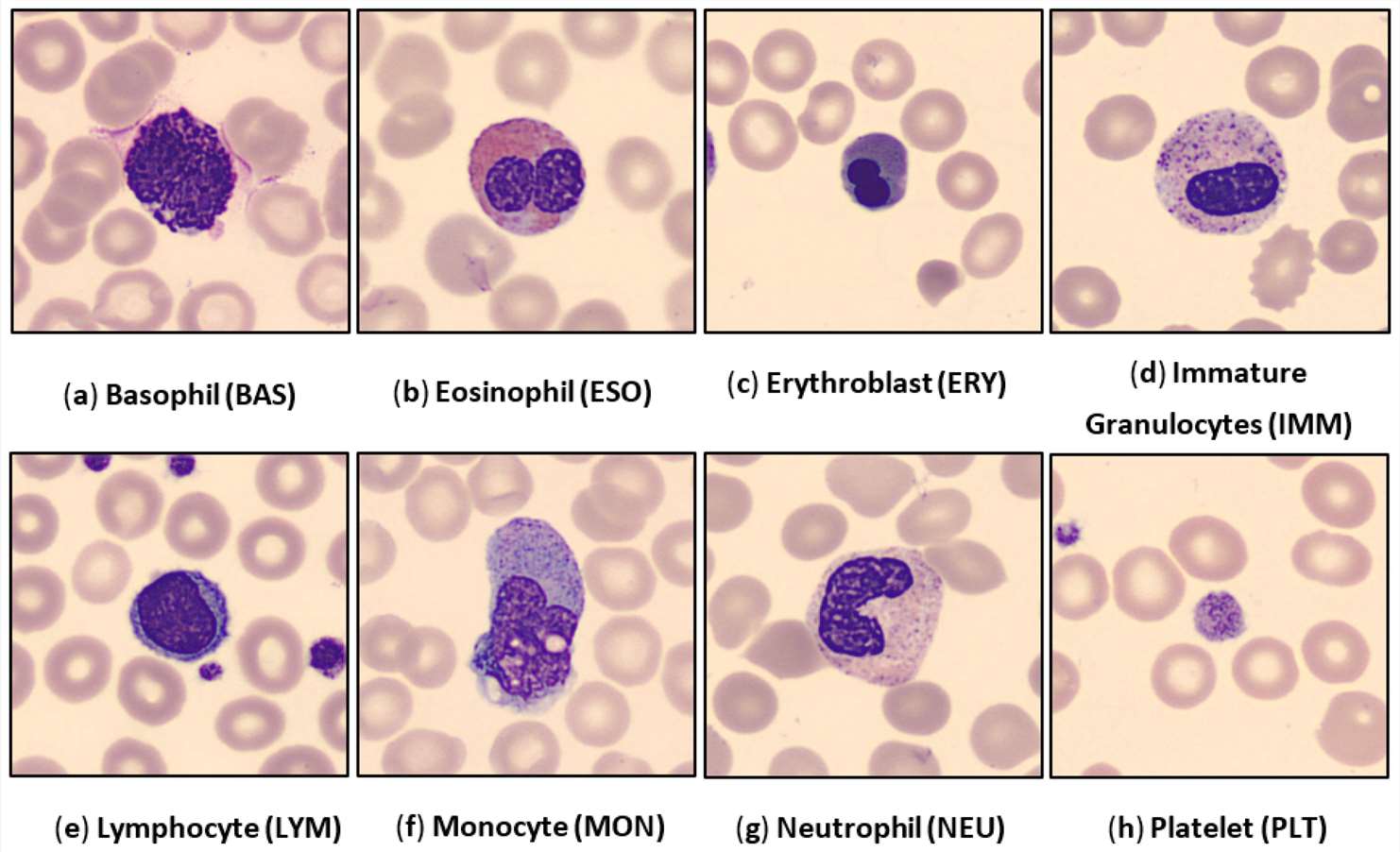 Fig. 1. Examples of the human peripheral blood cell (HPBC) images over all eight classes (Chola C, Muaad AY, et al., 2022).
Fig. 1. Examples of the human peripheral blood cell (HPBC) images over all eight classes (Chola C, Muaad AY, et al., 2022).
mCRP-Induced Monocyte Aggregation, Which was Concomitant with Increased Expression of FAK and M1 Phenotype Transition
Inflammation is a critical trigger for atherosclerosis, and C-reactive protein (CRP), particularly its dissociated monomeric form (mCRP), is significantly linked to disease progression. While the direct effects of mCRP on endothelial cells have been demonstrated, the mechanisms of interaction with blood monocytes remain unclear. Pastorello et al. aimed to elucidate the effects of mCRP on blood monocytes, specifically focusing on its role in promoting monocyte aggregation and differentiation into M1 macrophages via focal adhesion kinase (FAK) pathways, and assesses the potential inhibitory effects of C10M (CRP dissociation/mCRP inhibitor) on these processes.
Using freshly isolated human peripheral macrophages, Pastorello et al. observed a mCRP-induced aggregation pattern after 24 h (Fig. 1). In the absence of mCRP, control cultured peripheral monocytes did not cluster (Fig. 1A). Adding the CRP dissociation inhibitor C10M had no effect on clustering or cell appearance (Fig. 1B). However, 24 h exposure to mCRP induced noticeable clustering (Fig. 1). Pre-incubation with C10M eliminated floating clusters, though small adherent clumps remained (Fig. 1). Using a FAK inhibitor (Y397) inhibited mCRP-induced clustering and adherence (Fig. 1E-F).
Then, they performed the molecular mechanisms of mCRP-induced aggregation and motility changes. Human peripheral monocytes treated with mCRP showed significantly higher p-FAK expression compared to control cells or those treated with the inhibitor C10M (Fig. 2A-D). Pre-incubation with C10M partially blocked mCRP-induced p-FAK expression and nuclear translocation, while the FAK inhibitor completely blocked this effect (Fig. 2E-G). The increase in FAK expression induced by mCRP was significant, and both C10M and the FAK inhibitor significantly reduced it (Fig. 2H). Subsequent analysis of cell surface markers for the monocyte-macrophage phenotype indicated that mpCR treatment resulted in significant changes in the M1 phenotype.
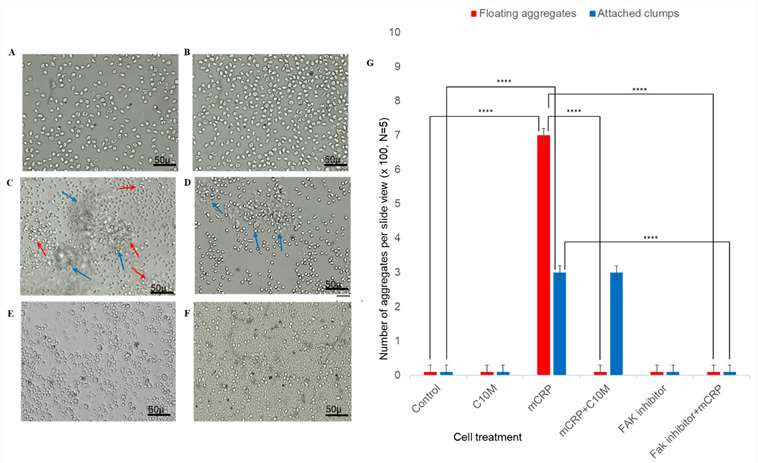 Fig. 1. mCRP-induced aggregation of peripheral blood monocytes (Pastorello Y, Manu D, et al., 2024).
Fig. 1. mCRP-induced aggregation of peripheral blood monocytes (Pastorello Y, Manu D, et al., 2024).
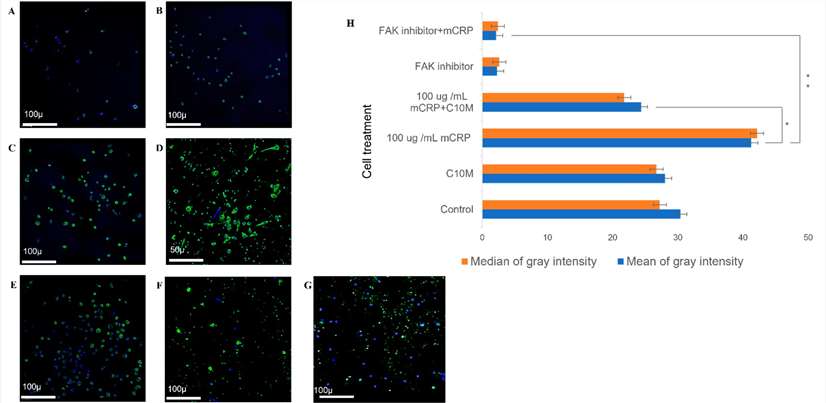 Fig. 2. Confocal analysis of FAK expression following mCRP treatment (Pastorello Y, Manu D, et al., 2024).
Fig. 2. Confocal analysis of FAK expression following mCRP treatment (Pastorello Y, Manu D, et al., 2024).
CX3CL1 Regulates the Differentiation of Human Peripheral Blood Monocytes into Osteoclasts.
It has been thought that peripheral blood monocytes migrate into synovial tissue of rheumatoid arthritis (RA) and differentiate into osteoclasts. Since osteoclasts are mainly involved in bone destruction, further studies are needed on the differentiation and activation of osteoclasts to clarify the pathogenesis of RA.
Chemokine (C-X3-C motif) ligand 1 (CX3CL1), also known as fractalkine, has dual functions as an adhesion molecule and chemoattractant. A correlation has been reported between the serum level of soluble CX3CL1 and disease activity in RA patients, suggesting that CX3CL1 plays important roles in the pathogenesis of RA. Muraoka' team initially examined the effects of CX3CL1 on osteoclast differentiation from peripheral blood monocytes. Peripheral blood monocytes from healthy donors were divided into CD16+ and CD16- monocytes and then incubated with macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) for 7 days. Osteoclasts were identified as TRAP-positive MNC. As previously reported, CD16- monocytes differentiated into osteoclasts when stimulated with M-CSF and RANKL (Fig. 3). Moreover, osteoclast differentiation from CD16- monocytes was significantly increased by the stimulation with CX3CL1 in a dose-dependent manner (Fig. 3). On the other hand, CD16+ monocytes treated with M-CSF and RANKL did not differentiate into osteoclasts, even with CX3CL1 (Fig. 3).
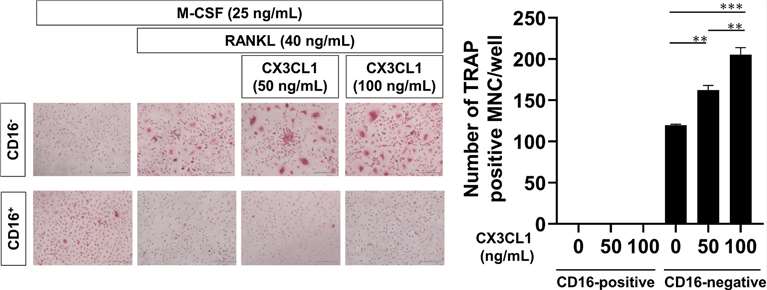 Fig. 3. Effects of CX3CL1 on the differentiation of peripheral blood monocytes into osteoclasts (Muraoka, S., Kaneko, K., et al., 2021).
Fig. 3. Effects of CX3CL1 on the differentiation of peripheral blood monocytes into osteoclasts (Muraoka, S., Kaneko, K., et al., 2021).
Ask a Question
Write your own review