ONLINE INQUIRY
C57BL/6 Mouse Dermal Microvascular Endothelial Cells
Cat.No.: CSC-C1871
Species: Mouse
Source: Dermis; Skin
Cell Type: Endothelial Cell; Microvascular Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
C57BL/6 Mouse Dermal Microvascular Endothelial Cells (mDMECs) were isolated from dermal tissue of the C57BL/6 mouse, an inbred strain that has gained a wide range of applications in immunology, oncology and metabolic disease. Such cells are tightly packed inside the linings of dermal microvessels, creating the endothelial layer of these blood vessels.
mDMECs can create and release a range of bioactive molecules that help regulate vasodilation and vasoconstriction, neovascularisation and tissue repair. Due to their strong angiogenic activity, mDMECs provide an indispensable platform to investigate signalling pathways for angiogenesis. In addition to all these roles, they are also involved in inflammation, immune systems and wound healing, which are essential to skin health and the management of skin diseases. Identified by endothelial cell markers, including von Willebrand Factor (vWF), CD31, Tie1 and Tie2, mDMECs also express a plethora of endothelial cell markers implicated in organogenesis. They encode proteins involved in the growth of organs, such as bone morphogenetic proteins-2 and-4 (BMP-2/-4) and their receptors (BMPR1A). In addition, they show good tolerance to a wide range of stimuli and interventions, and are suitable for a variety of experimental manipulations ranging from gene transfection to microinjection.
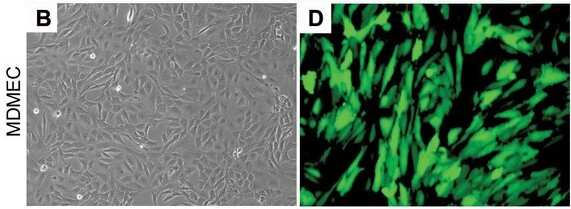 Fig. 1. Representative photomicrographs of mouse dermal microvascular endothelial cells (MDMEC) stably transduced with GFP (Dong Z, Imai A, et al., 2013).
Fig. 1. Representative photomicrographs of mouse dermal microvascular endothelial cells (MDMEC) stably transduced with GFP (Dong Z, Imai A, et al., 2013).
PEDF Induces Apoptosis and Inhibits Migration Of MDMEC, and Inhibits Endothelial Cell Tube Formation
Wound healing is a complex biological process, particularly characterized by angiogenesis, where increased capillary density forms an immature network. While the inflammatory and proliferative phases are well-understood, the regulation of the resolution phase remains unclear. The anti-angiogenic capability of PEDF has been best described in tissues other than skin, yet the mechanisms by which PEDF might influence capillary reduction in skin wounds are not yet well studied.
Michalczyk's team investigated the effect of PEDF on migration, tube formation, and the expression of key endothelial cell receptors by primary mouse dermal microvascular endothelial cells (MDMEC). Previous studies have shown that PEDF induces apoptosis in endothelial cells from non-skin tissues. To explore this in skin, MDMECs were treated with PEDF, resulting in apoptosis rates comparable to camptothecin (Fig. 1A). PEDF significantly increased early apoptosis (Annexin V+/PI+) (14% PEDF, 19% CPT, 4% control) (Fig. 1A). Moreover, PEDF treatment upregulated apoptosis markers Fas and caspase 3 (Fig. 1B). In vitro, PEDF inhibited MDMEC migration in a dose-dependent manner (Fig. 1C) and increased cell adhesion, possibly reducing migration (Fig. 1D). These findings align with PEDF effects on endothelial cells from other tissues like the retina and prostate. The ability of EC to form tubes on Matrigel is a commonly used assay for angiogenic capability. MDMEC were placed on Matrigel in the presence or absence of PEDF, and tube formation was assessed at 2 and 4 hours. Treatment of MDMEC with PEDF led to significant reductions in total tube length, total tubes, total branching points, and total loops as compared to the untreated control at both 2 and 4 hours (p < 0.05–0.01) (Fig. 2A and B). These results indicate that PEDF induces apoptosis, and inhibits dermal endothelial cell migration and endothelial cell tube formation in vitro.
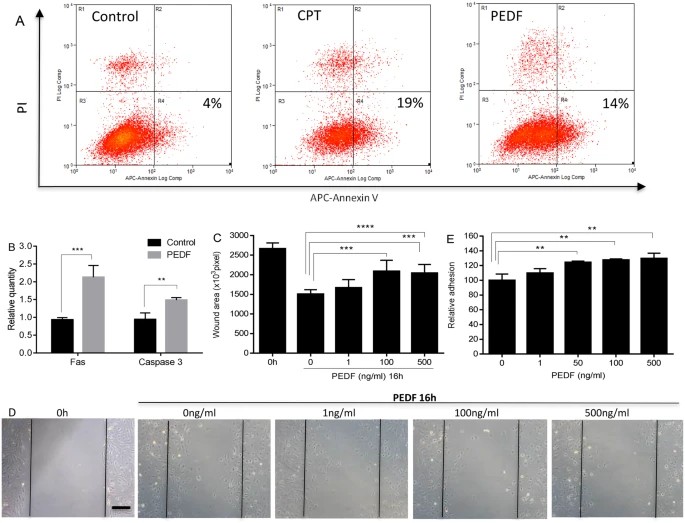 Fig. 1. Dose response effect of PEDF on apoptosis, migration, and adhesion of mouse dermal endothelial cells (MDMEC) (Michalczyk ER, Chen L, et al., 2018).
Fig. 1. Dose response effect of PEDF on apoptosis, migration, and adhesion of mouse dermal endothelial cells (MDMEC) (Michalczyk ER, Chen L, et al., 2018).
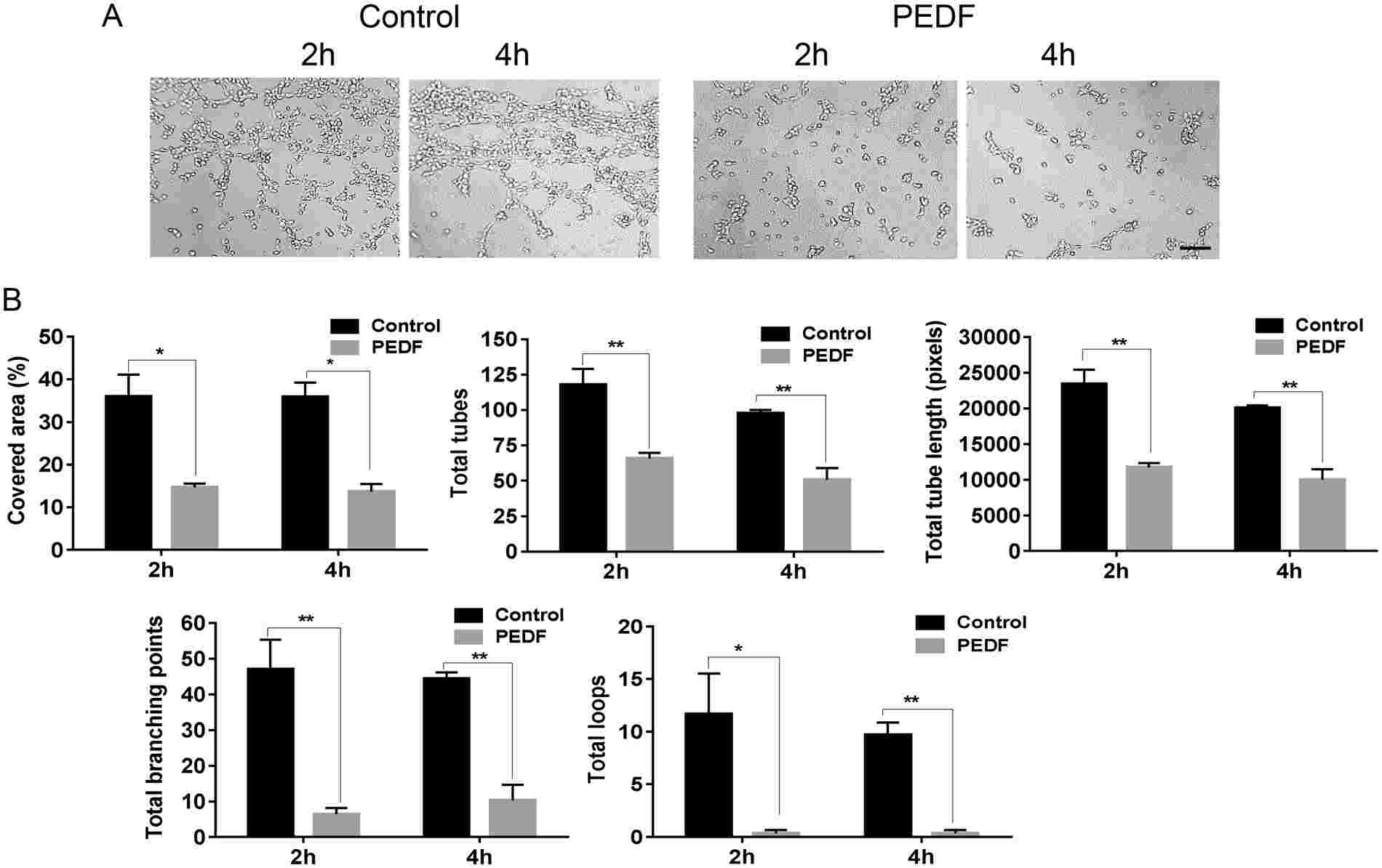 Fig. 2. Effect of PEDF on endothelial cell tube formation. MDMEC were cultured on Matrigel with or without PEDF (100 ng/ml) (Michalczyk ER, Chen L, et al., 2018).
Fig. 2. Effect of PEDF on endothelial cell tube formation. MDMEC were cultured on Matrigel with or without PEDF (100 ng/ml) (Michalczyk ER, Chen L, et al., 2018).
Phagocytosis of apoECs Leads to Altered Macrophage Phenotypes In Vivo
Wound healing is a complex physiological process involving macrophages, which play a crucial role in regulating inflammation and promoting tissue regeneration. Macrophages are known to influence angiogenesis and resolve excess vascular structures during wound healing by possibly engulfing apoptotic endothelial cells (ApoECs). Surprisingly few studies have investigated the fate of apoECs in wounds, or the consequence of their removal.
To examine the capability of macrophages to ingest apoECs in in vivo wounds, pHrodo green labeled apoECs were injected into skin wounds 6 days after injury. pHrodo green labeled apomECs were obtained by inducing apoptosis of C57BL/6 mouse primary dermal microvascular ECs (mECs) and co-incubation with pHrodo green. At 24 hours post-apoEC injection, macrophages in wound sites (CD68 marker) were identified to have ingested apoECs, as evidenced by double-positive yellow fluorescence (Fig. 3A, 3B). Flow cytometry revealed 2.2% of wound macrophages (F4/80+) contained labeled apoECs (Fig. 3C). Upon apoEC injection, M1 markers (CD80, CD86) in macrophages increased, while M2 marker CD163 remained unchanged compared to PBS controls (Fig. 4). Specifically, 100% of apoEC-engulfing macrophages expressed CD80, 93.8% expressed CD86, and 22.8% expressed CD163, but none expressed CD206 (Appendix D). This indicates apoEC exposure induces macrophages to adopt a distinct phenotype with M1 and M2 features in vivo.
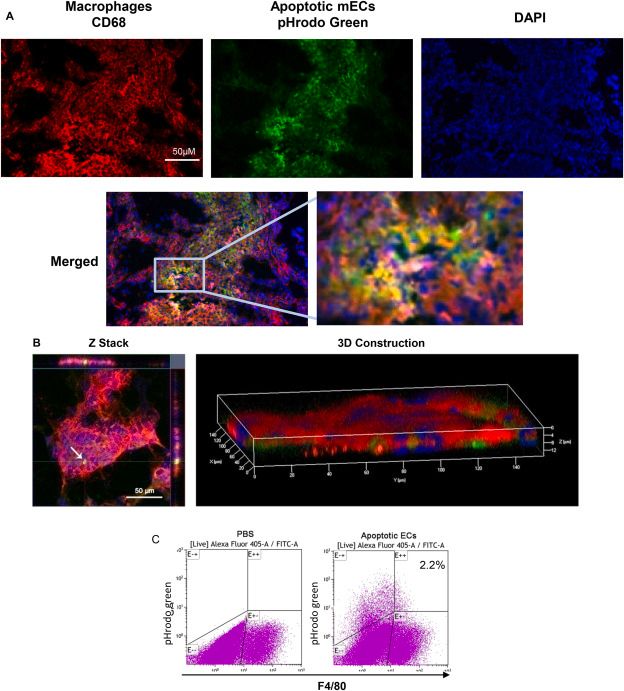 Fig. 3. Macrophages ingest apoptotic ECs in wounds in vivo.
Fig. 3. Macrophages ingest apoptotic ECs in wounds in vivo.
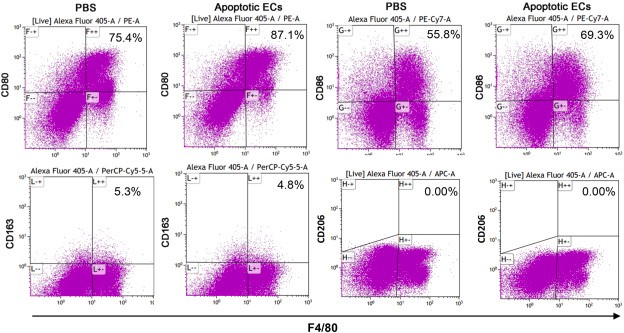 Fig. 4. Exposure to apoptotic dermal ECs alters macrophages phenotypes in vivo (Xu M, Chen Z, et al., 2021).
Fig. 4. Exposure to apoptotic dermal ECs alters macrophages phenotypes in vivo (Xu M, Chen Z, et al., 2021).
Ask a Question
Write your own review