ONLINE INQUIRY
ARPE-19
Cat.No.: CSC-C9083W
Species: Human
Source: Retina; Eye
Morphology: epithelial
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The ARPE-19 cell line is a retinal pigment epithelium (RPE) cell line first created in 1986 by Amy Aotaki-Keen. These cells were extracted from healthy ocular tissue from a 19-year-old man who had been killed in a car crash. By selectively trypsin-purging previous generations of cells out of the eye tissue, surface cells were then depolymerised into rapidly expanding RPE cells at generation 5. These cells began forming cobblestone-like monolayers in culture and were polarised in cell structure and function.
This retinal pigment epithelium (RPE) is a critical tissue between the retina and the choroid that serves many functions in maintaining normal retinal function including physiological functions like the visual circulation, transport of nutrients, and phagocytosis of the outer surfaces of photoreceptors.The ARPE-19 cell line mimics these functions and hence has a wide range of applications across ophthalmology, from drug screens to cell biology studies and 3D cell culture. Parallel to this, these cells produce retinal pigment epithelial markers like CRALBP and RPE-65, which makes them an ideal model for studying retinal pigment epithelium physiology and pathology. Additionally, the ARPE-19 cell line provides a perfect transfection host for gene function and drug effect experiments.
 Fig. 1. Morphology of ARPE-19 cells (Shanmuganathan S, Sumantran V N, et al., 2017)
Fig. 1. Morphology of ARPE-19 cells (Shanmuganathan S, Sumantran V N, et al., 2017)
Effects of Exogenous PGF on EMT in ARPE-19 Cells under Hypoxia
Age-related macular degeneration (AMD) is one of the leading causes of visual impairment from choroidal neovascularisation (CNV) that leads to subretinal fibrosis and scarring. Hypoxia contributes to AMD pathogenesis. PGF, one of the VEGF family members, promotes EMT, which may lead to retinal fibrosis. Subretinal fibrosis is a challenge in AMD control, even with anti-VEGF therapies. There's growing evidence that PGF may drive EMT and ultimately fibrosis. Zhang's team set out to understand how PGF contributes to ARPE-19 cell EMT under hypoxia.
First, the effect of exogenous PGF on the morphological changes of ARPE-19 cells under hypoxia was observed. Under hypoxia, the cells receiving PGF displayed marked cell morphology changes: a shift in morphology from epithelial to mesenchymal; the cells morphologically became longer and spindle-like and organised in an amorphous fashion. No clear morphological shift was detected in the cells exposed to hypoxia or to PGF alone (Fig. 1A-D). The MTT test indicated that: cell growth rate was remarkably reduced during CoCl2-induced hypoxia. The cells' proliferation rate was not affected by either exogenous PGF or not. Yet exogenous PGF induced an increased proliferation of the ARPE-19 cells in hypoxia compared with those in hypoxia alone (Fig. 1E-G). The wound healing assay revealed that ARPE-19 cells exposed to PGF and hypoxia had statistically significant accelerated migration. Neither hypoxia nor PGF alone significantly affected ARPE-19 cells' migration capability (Fig. 1H-I). ARPE-19 cell EMT was determined by qRT-PCR, western blotting, and immunofluorescence staining of EMT biomarkers at mRNA and protein levels. In cells exposed to hypoxia with PGF, the epithelial markers E-cadherin and ZO-1 were decreased, and the mesenchymal marker -SMA was elevated (Fig. 2), which suggested the occurrence of EMT, but neither exogenous PGF nor hypoxia exposure alone promoted EMT.
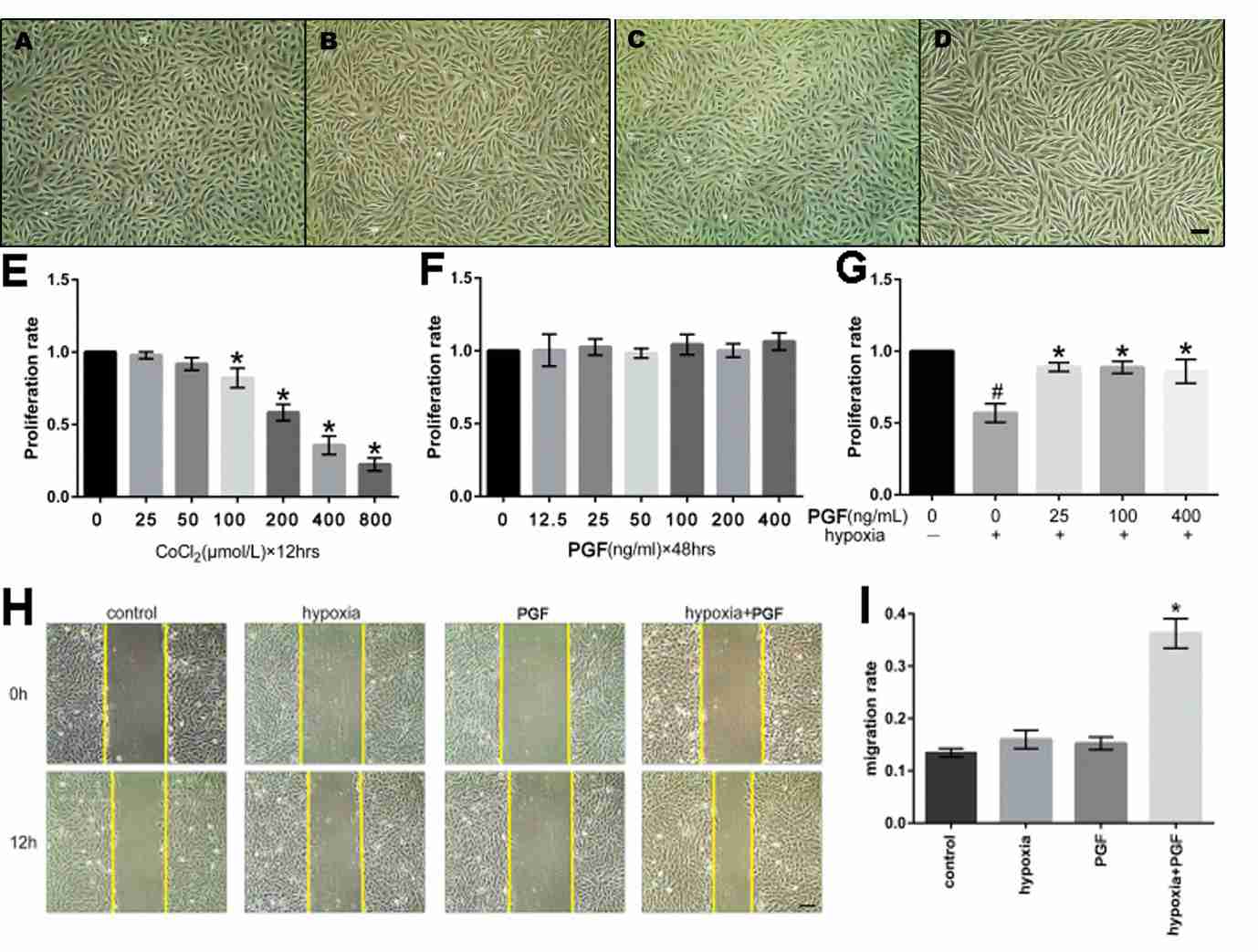 Fig. 1. (A-D) Effects of exogenous PGF on morphological changes of ARPE-19 cells under hypoxia. (E-G) Effects of hypoxia and PGF on the proliferation of ARPE-19 cells. (H-I) Effects of exogenous PGF on migration in ARPE-19 cells under hypoxia (Zhang Y, Zhao L, et al., 2018).
Fig. 1. (A-D) Effects of exogenous PGF on morphological changes of ARPE-19 cells under hypoxia. (E-G) Effects of hypoxia and PGF on the proliferation of ARPE-19 cells. (H-I) Effects of exogenous PGF on migration in ARPE-19 cells under hypoxia (Zhang Y, Zhao L, et al., 2018).
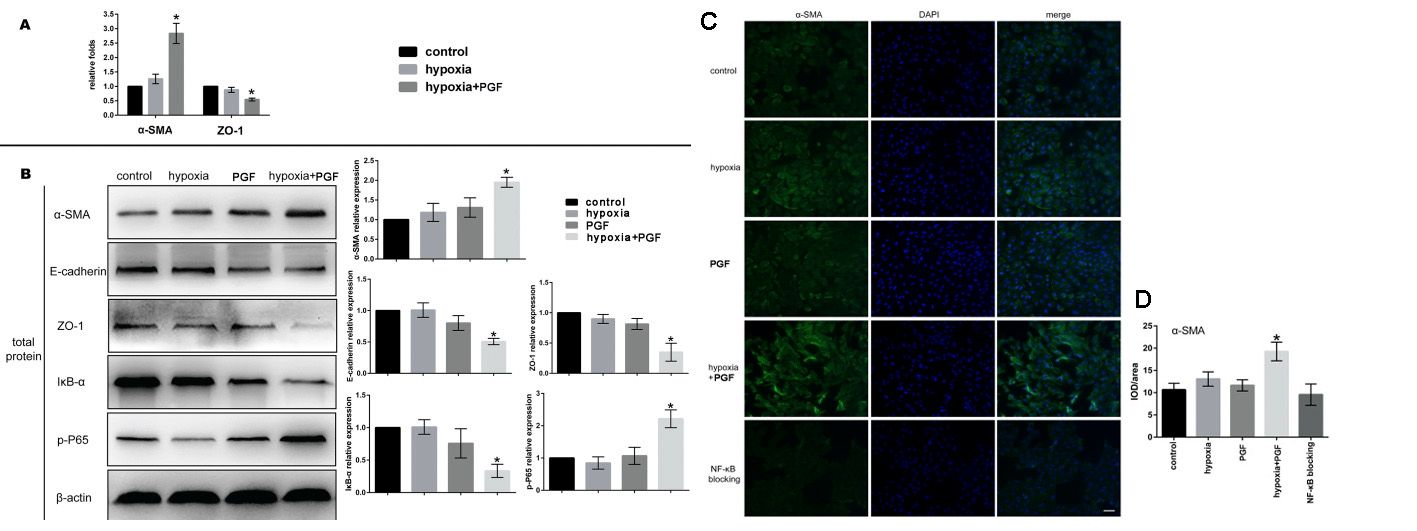 Fig. 2. Effects of exogenous PGF on EMT in ARPE-19 cells under hypoxia (Zhang Y, Zhao L, et al., 2018).
Fig. 2. Effects of exogenous PGF on EMT in ARPE-19 cells under hypoxia (Zhang Y, Zhao L, et al., 2018).
Antioxidative Effect of Astaxanthin and Ascorbic Acid by Reducing Intracellular ROS in ARPE-19 Cells
Oxidative stress contributes to DR and other retinal diseases by aggravatering retinal microvascular injury through production of reactive oxygen species (ROS). The antioxidative protections of the body, against free radicals and oxidative stress, are the key to reducing these effects. But age reduces endogenous antioxidants, debilitating this immunity.
Oh's team tested the antioxidative properties of ascorbic acid and astaxanthin in ARPE-19 cells in a well-characterized oxidative stress model, incorporating hydrogen peroxide and UVB light as ROS sources. The effects of ascorbic acid on ARPE-19 cells' intracellular ROS were evaluated with the DCFH-DA assay. After UVB (20 mJ/cm2 and 100 mJ/cm2), ROS levels climbed to 123% and 234%, respectively, while 500 M ascorbic acid decreased them to 105% and 115% (Fig. 3A), as observed by fluorescence microscopy (Fig. 3B). In cells incubated with H2O2 (0.2 mM and 0.4 mM), ROS levels rose to 123% and 135%, while ascorbic acid reduced them to 33% and 34% (Fig. 4A). When cells were treated with 20 M astaxanthin, 90 M ascorbic acid, or both, when they were exposed to 0.2 mM H2O2 for 24h, cell viability rose from 75% to 97%, 93%, and 129%, respectively (Fig. 4B). Both treatments also decreased ROS levels; 20 M astaxanthin and 90 M ascorbic acid dropped it to 169% and 135%, respectively, while the combination dropped it to 104% (Fig. 4C).
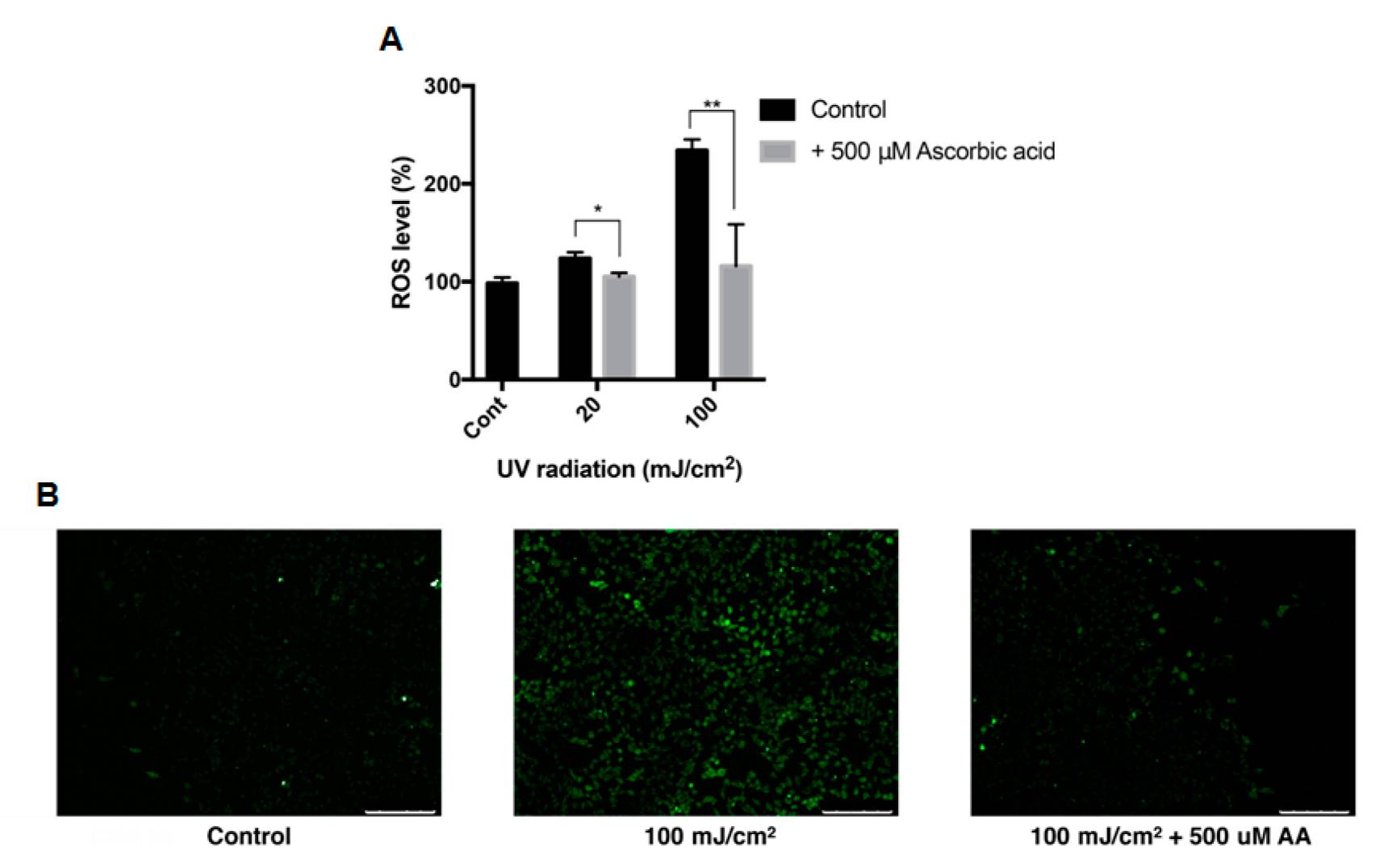 Fig. 3. Intracellular ROS level of ARPE-19 after UVB treatment with ascorbic acid (Oh S, Kim YJ, et al., 2020).
Fig. 3. Intracellular ROS level of ARPE-19 after UVB treatment with ascorbic acid (Oh S, Kim YJ, et al., 2020).
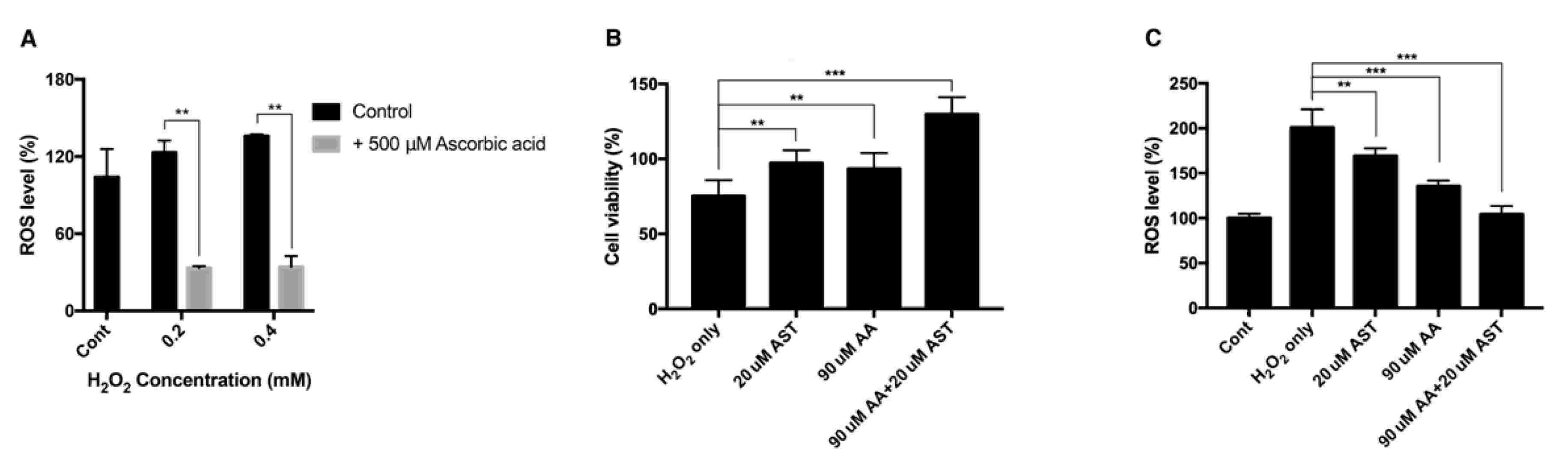 Fig. 4. Intracellular ROS level and cell viability of ARPE-19 after H2O2 exposure with ascorbic acid and the mixture of ascorbic acid and astaxanthin (Oh S, Kim YJ, et al., 2020).
Fig. 4. Intracellular ROS level and cell viability of ARPE-19 after H2O2 exposure with ascorbic acid and the mixture of ascorbic acid and astaxanthin (Oh S, Kim YJ, et al., 2020).
Ask a Question
Write your own review
- You May Also Need