Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Leukemia/Lymphoma/Myeloma Cells
- Product List
- Background
- Applications
- Scientific Data
- FAQ
Leukemia, lymphoma, and myeloma are a group of blood-related cancers that originate from the abnormal proliferation and differentiation of various types of blood cells. These malignancies can be broadly classified into acute and chronic forms, each with distinct characteristics.
Leukemia, for instance, is characterized by the uncontrolled growth of immature blood cells, often disrupting the normal function of the hematopoietic system. Lymphoma, on the other hand, involves the abnormal proliferation of lymphocytes, which can lead to the formation of solid tumors in the lymphatic system. Myeloma, a less common but equally devastating form of blood cancer, arises from the unregulated growth of plasma cells, a specialized type of white blood cell.
- Leukemia cells. One of the hallmarks of leukemia is the remarkable heterogeneity observed within the population of cancer cells. Each leukemia case can present with a unique genetic and phenotypic profile, reflecting the diverse mechanisms underlying the disease. Leukemia cells can be identified by their appearance under a microscope. The morphological features can vary based on the type of leukemia. In acute leukemias, the bone marrow and blood contain a high number of immature cells called blasts. These cells are larger than normal and have a high nucleus-to-cytoplasm ratio. In chronic leukemias, the abnormal cells are more mature but still function abnormally. Chronic lymphocytic leukemia (CLL) cells often resemble normal lymphocytes but are clonal and dysfunctional, while chronic myeloid leukemia (CML) cells typically include a mix of mature and immature myeloid cells.
- Lymphoma cells. Lymphoma can be broadly categorized into two main types: Hodgkin lymphoma (HL) and non-HL (NHL). Lymphoma cells are abnormal lymphocytes that multiply uncontrollably. Unlike normal lymphocytes, lymphoma cells do not undergo programmed cell death (apoptosis) and can accumulate in lymph nodes, spleen, and other tissues, disrupting normal immune function.
- Myeloma cells. Myeloma cells are malignant plasma cells that exhibit several distinctive characteristics. These abnormal cells can proliferate extensively within the bone marrow and produce large amounts of abnormal antibodies known as monoclonal proteins or M-proteins. The accumulation of these cells and proteins can cause damage to various organs and tissues.
Cancer Research
Leukemia, lymphoma, and myeloma cells are widely used as model systems to study the molecular mechanisms underlying the development and progression of different types of blood cancers. Researchers analyze the genetic, epigenetic, and signaling pathways involved in the transformation and proliferation of these cancer cells, which helps in the development of new targeted therapies.
Immunotherapy Research
Leukemia, lymphoma, and myeloma cells are employed in the development and testing of immunotherapies, such as immune checkpoint inhibitors and adoptive cell therapies. These cancer cells are used to understand the interactions between the immune system and blood cancers, as well as to evaluate the efficacy of immunotherapeutic approaches.
Drug Discovery and Testing
Leukemia, lymphoma, and myeloma cell lines are extensively used in high-throughput screening to identify new drug candidates and test their efficacy against these blood cancers. These cell lines help researchers assess the cytotoxicity, selectivity, and mechanism of action of potential anti-cancer drugs.
Leusin-1 ArrestsLeukemia in G2-phase
To determine the consequences of arresting cells in the G2 phase with Leusin-1, the biochemical response of cells treated with Leusin-1. CCRF-CEM cells were treated with Leusin-1, cisplatin (G2-phase inhibitor), or Taxol (M-phase inhibitor), and protein extracts were prepared after 24 h. Consistent with our previous data, immunoblot analyses of protein samples using antibodies directed against p-H3 (phosphorylated in mitosis) indicated that Leusin-1 and cisplatin arrested cells with limited p-H3 staining, indicative of a G2-phase arrest, whereas Taxol arrested cells with increased p-H3 levels, indicative of an M-phase arrest (Fig. 1A). Interestingly, Leusin-1 induced the cleavage of caspase-3, indicative of apoptotic pathway activation (Fig. 1A). These data indicated that Leusin-1 arrested cells before mitosis and triggered apoptotic cell death. To further test this, CCRF-CEM cells were treated with DMSO, Leusin-1, or Taxol for 48 h and the extent of caspase-3/7 activation was measured using the Caspase-Glo luminescent caspase activity assay. This assay revealed that Leusin-1 was indeed inducing an apoptotic cell death similar to Taxol treatment (Fig. 1B). Next, live-cell time-lapse microscopy was performed on CCRF-CEM cells treated with DMSO or Leusin-1. DMSO-treated cells were able to divide normally, whereas Leusin-1-treated cells never divided and eventually underwent apoptosis (Fig. 1C). Together these data indicated that Leusin-1 was arresting cells in G2-phase and triggering apoptotic cell death.
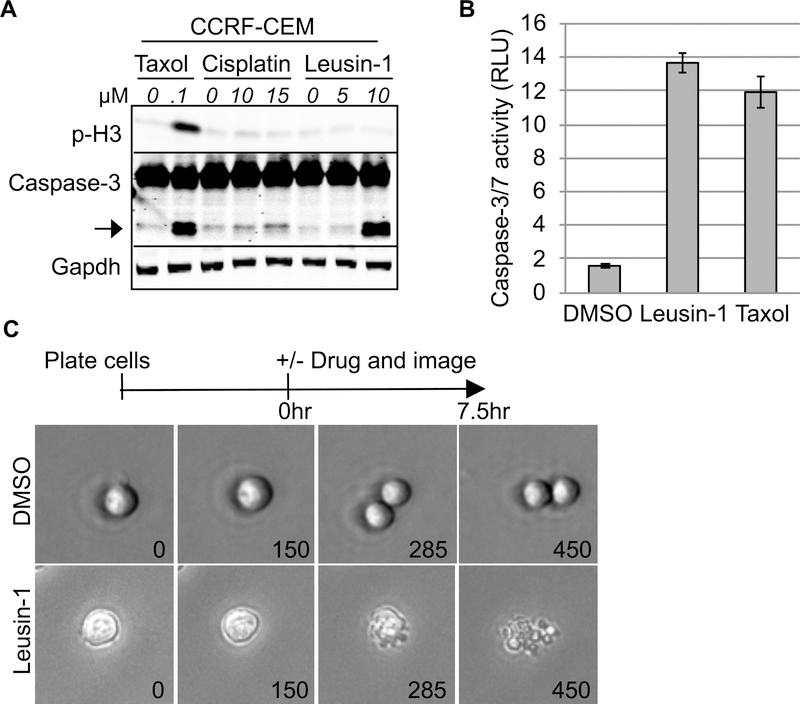 Fig. 1 Leusin-1 arrests cells in the G2 phase and triggers apoptotic cell death. (A) CCRF-CEM cells were treated with DMSO or the indicated concentrations of Leusin-1, Taxol, or cisplatin for 24 h. (B) CCRF-CEM cells were treated with DMSO, Leusin-1 (5 μM), or Taxol (100 nM) for 48 h and the caspase-3/7 activity was quantified using the Caspase-Glo luminescent caspase activity assay. (C) Live-cell time-lapse microscopy of CCRF-CEM cells treated with DMSO or Leusin-1 (5 μM). (Xia X, et al, 2019)
Fig. 1 Leusin-1 arrests cells in the G2 phase and triggers apoptotic cell death. (A) CCRF-CEM cells were treated with DMSO or the indicated concentrations of Leusin-1, Taxol, or cisplatin for 24 h. (B) CCRF-CEM cells were treated with DMSO, Leusin-1 (5 μM), or Taxol (100 nM) for 48 h and the caspase-3/7 activity was quantified using the Caspase-Glo luminescent caspase activity assay. (C) Live-cell time-lapse microscopy of CCRF-CEM cells treated with DMSO or Leusin-1 (5 μM). (Xia X, et al, 2019)
ApoEVs Repress Multiple Myeloma Progression
Recently, emerging research reports that apoptotic extracellular vesicles (apoEVs) can transfer cellular components to cells in nearby or even distant tissues. To test the effect of mesenchymal stem cell (MSC)-derived apoEVs on multiple myeloma (MM) in vivo, an aggressive syngeneic MM mouse model was generated by tail vein infusion of 5TGM1 myeloma cells into NOD/SCID mice (Fig. 2A). This MM model showed typical myeloma-associated bone phenotypes, and the mice died 26 days after 5TGM1 cell injection. Systemic infusion of apoEVs significantly extended the life span of MM mice to an average of 40 days (Fig. 2B). Next, apoEV treatment dramatically repressed tumor growth at 14 and 25 days after luciferase expressing-5TGM1 tumor cell inoculation, as monitored by live animal bioluminescent imaging (Fig. 2C, D). Immunofluorescent staining and flow cytometric analysis further verified that increased numbers of infiltrated CD138+ plasma cells and CD45+CD19-CD138+ myeloma cells were detected in femur bone marrow in MM mice compared with control mice (Fig. 2E, F). ApoEV infusion dramatically repressed the pathological infiltrated myeloma cells in MM mice (Fig. 2E, F). Nanosized agents preferentially enter and enrich solid tumors via passive targeting due to vascular system leakage and lymphatic drainage defects, which is called the enhanced permeability and retention (EPR) effect.
Since abnormal proliferated myeloma cells produce excessive immunoglobulins, serum IgG is one of the preferred markers to monitor MM activity in patients. Enzyme-linked immunosorbent assay (ELISA) analysis revealed that apoEV treatment markedly reduced the elevated IgG2b levels in MM mice (Fig. 2G). MicroCT and histological analysis displayed that the trabecular bone mineral density (BMD) and trabecular bone volume fraction (bone volume/trabecular volume, BV/TV) decreased at 25 days after 5TGM1 cell-inoculation (Fig. 2H, I). ApoEV infusion markedly restored BMD and BV/TV in MM mice (Fig. 2H, I). In addition, histological analysis showed that MM mice exhibited severe acute kidney injury, indicated by tubulointerstitial nephritis and cast nephropathy, whereas apoEV treatment significantly rescued the kidney injury (Fig. 2J). These data indicate that MSC-derived apoEVs can inhibit MM cell growth and extend the survival of MM mice. Preclinical data suggest MSC-EV-based therapy may serve as a cell-free alternative for inflammatory diseases.
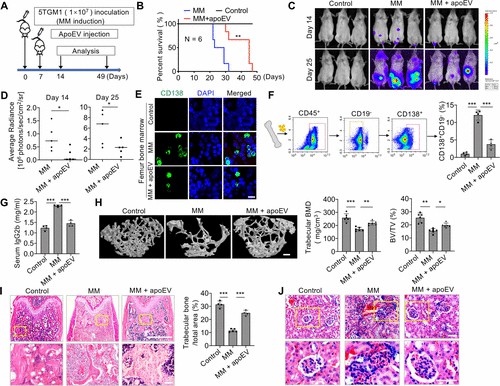 Fig. 2 Mesenchymal stem cell-derived apoEVs inhibit MM cell growth. (Wang J, et al., 2021)
Fig. 2 Mesenchymal stem cell-derived apoEVs inhibit MM cell growth. (Wang J, et al., 2021)
The main types of blood cancers that heavily utilize leukemia, lymphoma, and myeloma cells in research include acute myeloid leukemia, CML, acute lymphoblastic leukemia, non-HL, and HL.
The key advantages of using these cell lines include an unlimited and consistent supply of cancer cells for experiments, the ability to study the biology and behavior of cancer cells in a controlled environment, facilitating high-throughput screening of drug candidates and evaluation of therapeutic responses, etc.
Researchers employ various quality control measures to ensure the authenticity and quality of these cell lines, such as periodic DNA profiling or short tandem repeat (STR) analysis, mycoplasma testing, and monitoring of cell morphology, growth characteristics, and marker expression.
Description: The NAMALWA.CSN/70 cell line is a subclone of the cell line NAMALWA. Established...
Description: Established from the tumor mass of an African child with Burkitt lymphoma; this variant...
Description: Established from the bone marrow of a 20-year-old woman with Hodgkin lymphoma (nodular...
Description: Established from the ascites fluid of a patient with American-type Burkitt lymphoma...
Description: Established from the pleural effusion of a patient with B cell lymphoma (undifferentiated...
Description: Established from the pleural effusion of a 10-year-old boy with Burkitt lymphoma...
Description: Established from the peripheral blood of a 62-year-old man with Philadelphia chromosome...
Description: Established from the peripheral blood of a 6-month-old girl with acute megakaryoblastic...
Description: Established from the peripheral blood of a 19-year-old man with acute lymphoblastic...
Description: Established from the bone marrow of a 5-year-old Caucasian girl with Philadelphia...
Description: Established from the bone marrow of a 5-year-old Caucasian girl with Philadelphia...
Description: established from the bone marrow of a 64-year-old man with acute myeloid leukemia...
Description: Established from the peripheral blood of a 20-year-old man with acute B cell leukemia...
Description: The SUP-T1 established from the pleural effusion of an 8-year-old boy with T-lymphoblastic...
Description: Supposedly "established from the peripheral blood of a 72-year-old woman with acute...
Description: Established from the tumor tissue of an 8-year-old Caucasian boy with Burkitt lymphoma...
Description: Established from the pleural effusion of a 37-year-old woman with Hodgkin lymphoma...
Description: B-lymphoblastoid cell line established from the peripheral blood of a 73-year-old...

