Overview of Exosomes
What Are Exosomes?
Exosomes are small, double-membrane lipid vesicles secreted by cells, which are usually between 30-150 nanometers in diameter. Exosomes can exist stably in various biological fluids, such as blood, urine, saliva and cerebrospinal fluid. In the intercellular participation in the transmission of information and material exchange, with important biological functions, such as regulating the immune response, cell proliferation and so on.
Structure and composition: Exosomes contents proteins, lipids, nucleic acids (mRNA, miRNA, ncRNA), etc. Their lipid bilayer is mainly composed of lipids such as cholesterol, sphingolipids, and phosphoglycerides. The surface also attached a large number of proteins, mRNAs, miRNAs, and non-coding RNAs (ncRNAs), etc.
Table. 1. Summary of exosomal compositions (Liang Y, Duan L, et al., 2021).
| Composition | Type |
| Proteins | cytosolic proteins, cell surface proteins, membrane-associated proteins,cytoskeletal proteins,enzymes |
| Lipids | sphingomyelins, phosphatidylserines, phosphatidylethanolamines, phosphatidylcholines, phosphatidylinositols, glycosphingolipid GM3, ceramides, fatty acids, glycerolipids, glycerophospholipids, sterol lipids, steroids |
| Nucleic acids | mRNAs, miRNAs, noncoding RNAs, mtDNAs |
| Metabolites | carboxylic acids, amino acids, sugars, carnitines, biogenic amines, vitamins, cyclic alcohols |
Exosome biogenesis is a complex process, and this process usually begins with the endosomal pathway. Generally, early endosomes form Multivesicular Bodies (MVBs) through invagination, and when these MVBs fuse with the cell membrane, the endosomal vesicles are released to the outside of the cell, and these endosomal vesicles are known as exosomes. The secretion process of exosomes is regulated by a variety of factors, including specific proteins, lipids and signaling pathways.
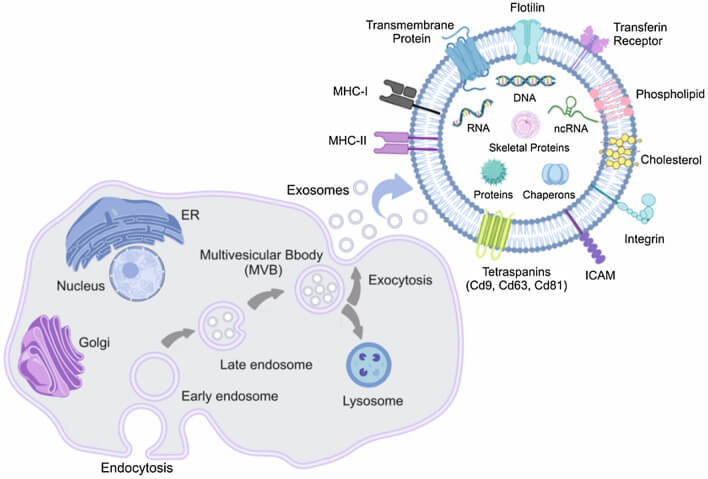 Fig.1. Biogenesis and composition of exosomes (Sadeghi S, Tehrani FR, et al., 2023).
Fig.1. Biogenesis and composition of exosomes (Sadeghi S, Tehrani FR, et al., 2023).
Exosome uptake:
After exosomes arrive at target cells, there are three main ways to act on the target cells
- Direct interaction with target cell plasma membrane receptors via surface-specific proteins.
- Fusion with the membrane.
- Target cells take up exosomes through endocytosis.
Current research suggests that endocytosis may be the most common mode, in which exosomes entering the target cell via endocytosis undergo an “endosomal escape” process into the cytoplasm, where they perform specific functions.
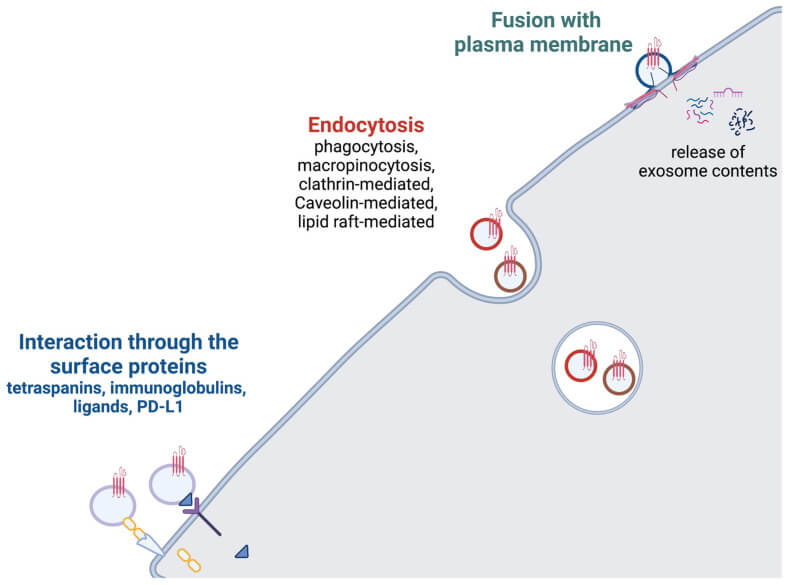 Fig. 2. Exosome uptake (Krylova SV, and Feng D, 2023).
Fig. 2. Exosome uptake (Krylova SV, and Feng D, 2023).
- Resource
- Service and Product
Exosomes Functions
Cellular communication: Exosomes communicate with other cells by releasing miRNAs, proteins and other active biomolecules. These signal molecules, when captured by target molecules, affect gene expression and protein synthesis of cells, thereby regulating their physiological and pathological states.
Immune regulation: By carrying immunomodulatory molecules, exosomes affect the activation, proliferation and differentiation of immune cells, thereby regulating inflammatory responses and promoting wound healing.
Tissue repair and regeneration: Exosomes contain bioactive molecules like growth factors and cytokines, which can enhance cell proliferation, migration, and differentiation, participating in the processes of tissue repair and regeneration.
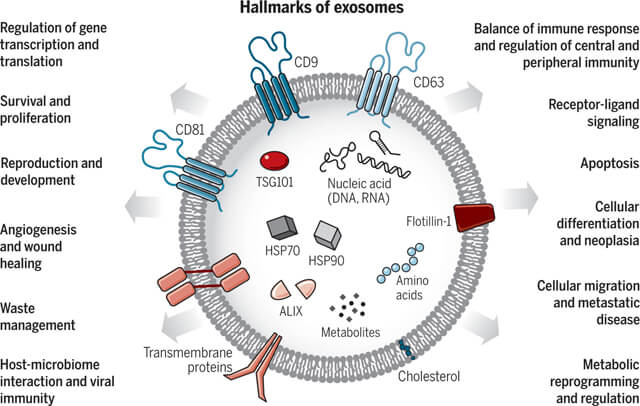 Fig. 3. Exosomes: A cell-to-cell transit system in the human body with pleiotropic functions (Kalluri R, LeBleu VS, et al., 2020).
Fig. 3. Exosomes: A cell-to-cell transit system in the human body with pleiotropic functions (Kalluri R, LeBleu VS, et al., 2020).
- Resource
- Service and Product
Exosome Study Techniques
Exosomes Isolation and purification technology:
Differential Pressure Ultracentrifugation (DPU) is the most classical method for exosome isolation, and has long been considered the “gold standard” for exosome isolation due to its high throughput.
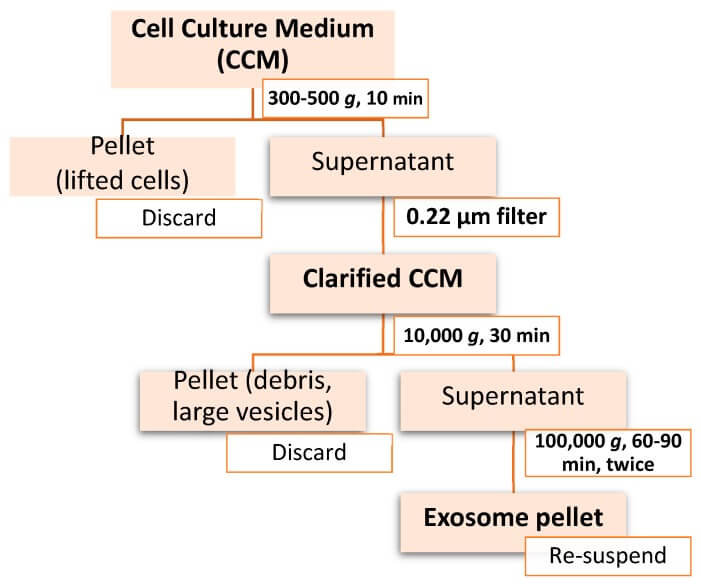 Fig. 4. Workflow of differential ultracentrifugation for exosome isolation (Doyle LM, Wang MZ, et al., 2019).
Fig. 4. Workflow of differential ultracentrifugation for exosome isolation (Doyle LM, Wang MZ, et al., 2019).
However, this method also has the disadvantage of protein and lipid contamination. With the progress of exosome separation technology, a variety of separation techniques have been developed by utilizing its different physicochemical and biochemical properties:
- Ultracentrifugation techniques: Differential ultracentrifugation, density gradient centrifugation,rate-zonal centrifugation, isopycnic centrifugation.
- Size based techniques: Ultrafiltration, exosome isolation kit, sequential filtration, size exclusion chromatography (SEC), flow field-flow fractionation (FFFF), hydrostatic filtration dialysis (HFD).
- Immunoaffinity capture-based techniques: Enzyme-linked immunosorbent assay (ELISA), magneto-immunoprecipitation.
- Exosome precipitation: Polyethylene glycol (PEG) precipitation, lectin induced agglutination.
- Microfluidic based isolation techniques: Acoustic nanofilter, immuno-based microfluidic isolation.
Table. 2. Current exosome isolation methods and their advantages and disadvantages.
| Method | Advantage | Disadvantage |
| Differential Ultracentrifugation |
|
|
| Density gradient UC |
|
|
| Ultrafiltration |
|
|
| Size‐exclusion chromatography |
|
|
| ||
| Immunoaffinity capture |
| |
| ||
| ||
| Polymer precipitation |
The identification of exosomes generally includes two aspects: the identification of morphological size and surface protein markers. The International Society for Extracellular Vesicles defined the minimal experimental requirements for exosome characterization in 2018:
- (1) Overall characterization: At least 3 positive protein markers (including at least 1 transmembrane protein and 1 cytosolic protein) and 1 negative protein marker.
- (2) Individual characterization: Utilize at least two different but complementary techniques.
Protein identification methods are mainly conventional protein immunoblotting, and individual characterization is mainly NTA and electron microscopy. In addition, nano-flow is a new method used for the characterization of exosomes, which is an upgrade of flow cytometry and allows higher resolution to identify exosomes in terms of particle size, concentration, purity and markers.
Exosome analysis
With more and more research on the function and application of exosomes, there are more and more requirements for their physicochemical properties and composition analysis.
Physical analyses of exosome, such as size and concentration, can be performed by techniques such as nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), electron microscopy, and tunable resistance pulse sensing (tRPS). Biochemical properties and compositional analyses are usually performed by staining or immunoblotting and proteomics techniques.
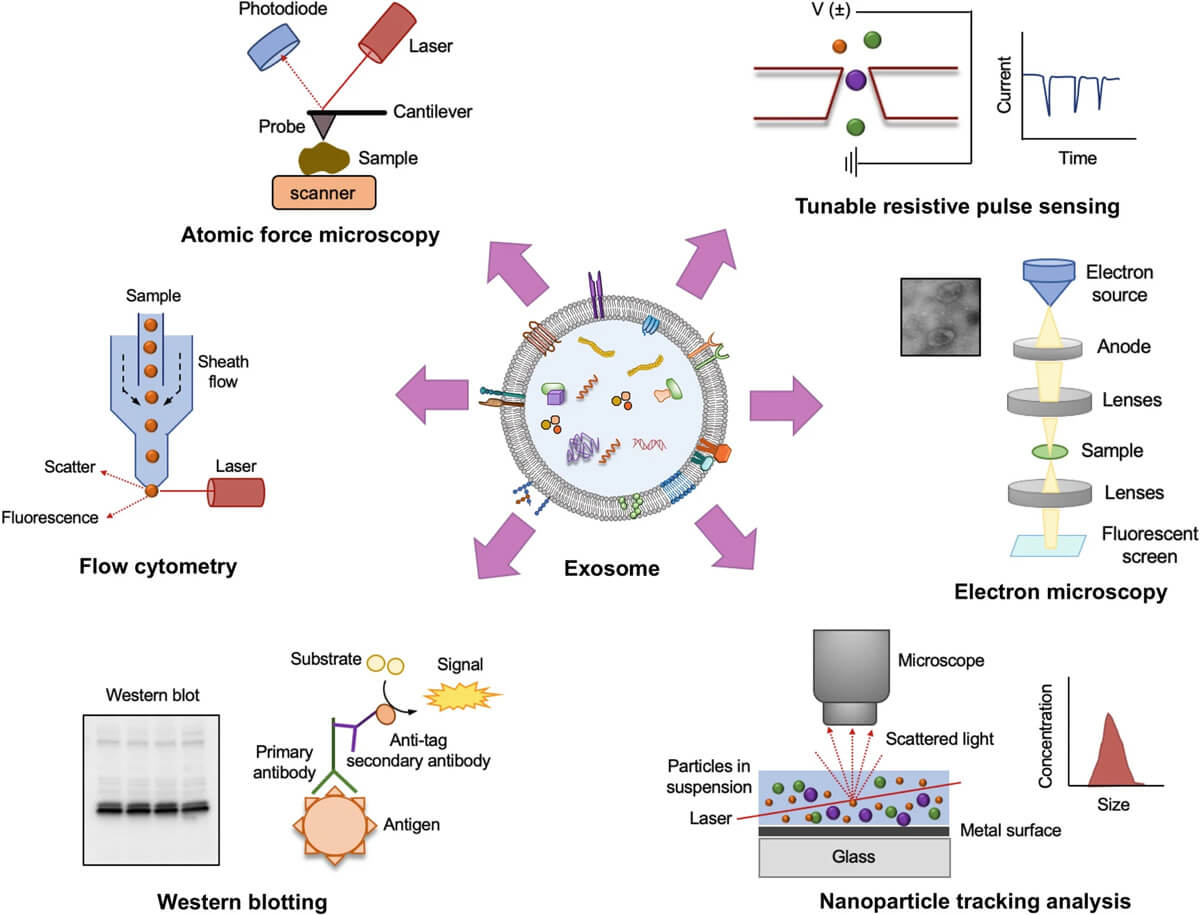 Fig. 5. Various methods of exosome characterization (Ranjan P, Verma S, et al., 2024).
Fig. 5. Various methods of exosome characterization (Ranjan P, Verma S, et al., 2024).
- Resource
- Service and Product
- Collection of Exosome Samples and Precautions
- Classification, Isolation Techniques and Characterization of Exosomes
- Techniques for Exosome Quantification
- Exosome Size Measurement
- Emerging Technologies and Methodologies for Exosome Research
- Common Techniques for Exosome Nucleic Acid Extraction
- Exosome Quality Control: How to Do It?
Exosome Engineering Techniques
Exosome transfection
The transfection technology of exosomes is mainly to introduce exogenous genetic material (e.g. plasmid DNA) or therapeutic molecules (e.g. proteins, nucleic acids, etc.) into exosomes, so as to utilize the characteristics of exosomes for intercellular information transfer or gene therapy. This is a crucial aspect of exosome research and application, significantly impacting regenerative medicine and drug delivery. Current exosome transfection techniques can be divided into:
- Physical methods: ultrasound, electroporation, freeze-thaw, extrusion;
- Chemical methods: saponin-assisted infiltration, transfection reagent-based embedding
- Biological: incubation and viral transduction, etc.
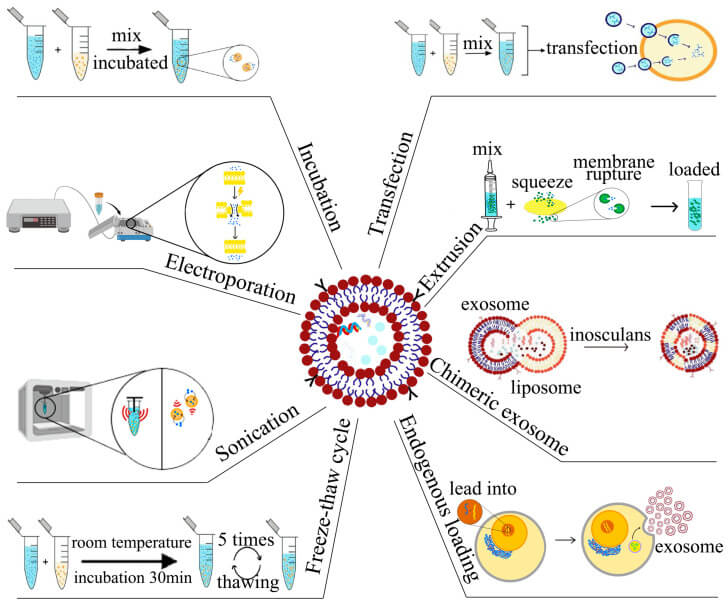 Fig. 6. Strategies for exosome drug loading (Zeng H, Guo S, et al., 2023).
Fig. 6. Strategies for exosome drug loading (Zeng H, Guo S, et al., 2023).
Targeted modifications
Exosomes are widely involved in a variety of physiological processes such as cellular immune response, antigen presentation, cell migration, cell differentiation, tumor invasion, etc. Extracellular vesicles in their unmodified state possess a certain degree of targeting ability, though it is relatively low. When used as therapeutics and drug delivery vehicles, it is necessary to enhance their targeted delivery capabilities through genetic engineering or surface modification of the membrane. Genetic engineering techniques include gene fusion and gene editing (CRISPR-Cas9, ZFN and TALEN, etc.), and membrane modifications can be categorized into:
- Chemical modifications: lipid modification, chemical coupling and click chemistry.
- Physical modifications: electrostatic interactions, hydrophobic interactions/membrane fusion, magnetic guidance, acoustic guidance, metal particle modifications.
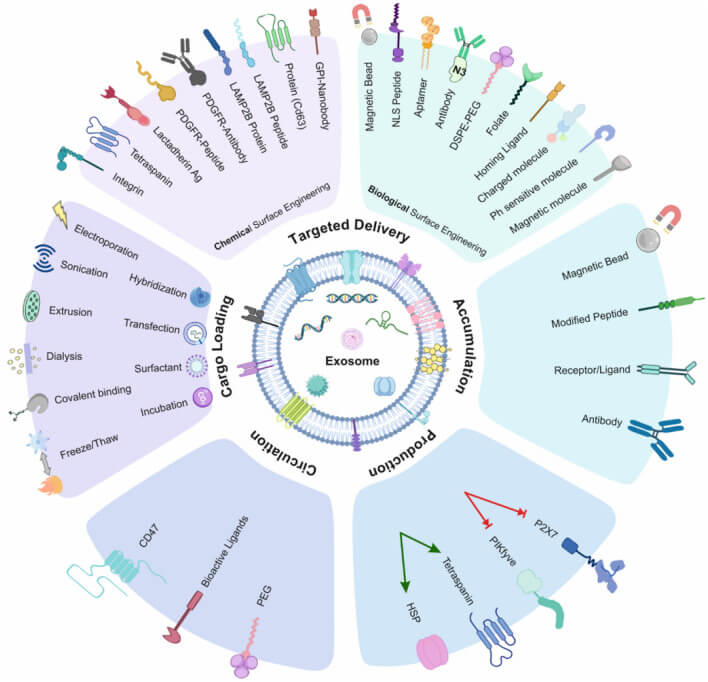 Fig. 7. Summary of exosome modification methods (Sadeghi S, Tehrani FR, et al., 2023).
Fig. 7. Summary of exosome modification methods (Sadeghi S, Tehrani FR, et al., 2023).
Labeling techniques:
Exosome labeling technology facilitate separation, identification and tracking of the source, structure and biological function of exosomes. According to the difference in exosomes size with other cellular debris, the accurate labeling and identification of exosomes is the basic and important step for research. In the study of cellular communication and signaling mechanism, exosome labeling technology is helpful to disclose exosome transportation path and target cell, which is widely used to investigate the origin of exosome and disease mechanism. So far, the labeling technique of exosome can be divided into three categories.
- Chemical labeling: lipophilic dyes (PKH and lipophilic carbocyanine dyes), permeable dyes (e.g., carboxyfluorescein diacetate succinimidyl ester (CFDA), calcein-AM), and near-infrared (NIR) fluorescent probes.
- Physical labeling: quantum dots (QDs), radiolabeling, superparamagnetic iron oxide nanoparticles (SPION), and gold nanoparticle (GNP) labeling.
- Biological methods: fluorescent protein fusion expression, lentivirus-mediated expression, and luciferase labeling.
Over the years, labeling techniques of exosomes have grown invariably.There are several distinct advantages and drawbacks regarding diverse labeling techniques of exosomes. It is imperative to select the suitable labeling pratice according to the requirement of the research. A proper technique selection is the key to the sucess of the experiement.
- Resource
- Service and Product
Exosomes Applications
Disease diagnosis: Exosomes present in blood, urine, saliva or cerebrospinal fluid are able to serve as non-invasive or minimally invasive biomarkers for disease diagnosis, and are capable of detecting many pathological conditions, such as cancers, cardiovascular diseases and neurodegenerative disorders.
Drug delivery: RNA or small molecules encapsulated in exosomes can cross the blood vessel wall and extracellular matrix, evading phagocytosis by monocytes. As such, exosomes represent a new platform for delivery of RNA interference agents and other therapeutics (eg, lipophilic small molecules) into cancer cells. They also represent a promising new form of drug delivery owing to their endogenous nature, which limits potential issues of immunogenicity and toxicity.
Regenerative medicine: The molecules packaged in exosomes can stimulate cell proliferation and differentiation, and thus provide ideal candidates for improving tissue repair and regenerative medicine.
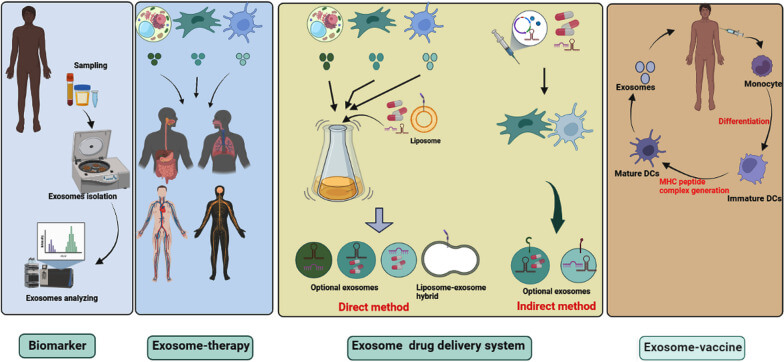 Fig. 8. Clinical application of exosomes. In clinical trials, exosomes are being used as biomarkers, cell-free therapy (Rezaie J, Feghhi M, et al., 2022).
Fig. 8. Clinical application of exosomes. In clinical trials, exosomes are being used as biomarkers, cell-free therapy (Rezaie J, Feghhi M, et al., 2022).
- Resource
- Service and Product
- Applications of MSC-EVs in Immune Regulation and Regeneration
- Exosome Antibodies
- Exosomes as Emerging Biomarker Tools for Diseases
- Current Research Status of Milk Exosomes
- The Role of Exosomes in Cancer
- How to Efficiently Utilize MSC Exosomes for Disease Treatment?
- How to Apply Exosomes in Clinical?
- Production of Exosomes: Human Cell Lines and Cultivation Modes
- How do PELN Deliver Drugs?
- What’s the Potential of PELN in Disease Treatment?
- How to Enhancement Exosome Production?
References
- Liang Y, Duan L, et al. Engineering exosomes for targeted drug delivery. Theranostics. 2021. 11(7):3183-3195.
- Krylova SV, Feng D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int J Mol Sci. 2023. 24(2):1337.
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. 367(6478):eaau6977.
- Sadeghi S, Tehrani FR, et al. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology. 2023. 31(1):145-169.
- Ranjan P, Verma SK. Exosomes Isolation, Purification, and Characterization. Methods Mol Biol. 2024. 2835:173-180.
- Zeng H, Guo S, et al. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells. 2023. 12(10):1416.
- Rezaie J, Feghhi M, et al. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022. 20(1):145.
- Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019. 8(7):727.

