How to Label Exosomes?
Exosomes are cell-secreted extracellular vesicles, which range in size from 30 to 150 nm. They can carry various bioactive molecules, including RNA, lipids and proteins, to mediate intercellular communication. However, the precise roles of exosomes in vivo remain unclear. We now have several ways to label exosomes and study their behaviour in living cells. The article summarizes popular exosome tracking methods and their characteristics.
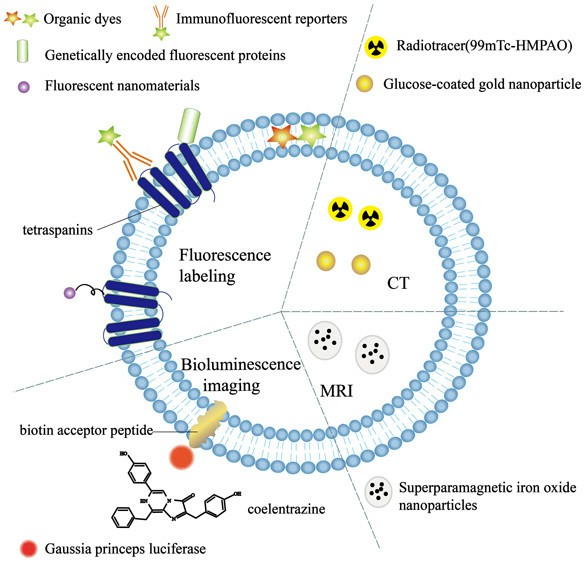 Fig. 1. Exosomes labeling method (Shen L M., Quan L L., et al., 2018).
Fig. 1. Exosomes labeling method (Shen L M., Quan L L., et al., 2018).
Chemical Labeling Method
Lipophilic dyes
Fluorescent membrane dyes with intense fluorescent signals when their aliphatic tails are placed in the lipid bilayer of exosomes. Lipid dyes, which are more often found in the literature, are widely used for the labeling of exosomes in both the animal and the cell. These dyes come in two main types:
- PKH: PKH dyes are fluorescent molecules with long aliphatic tails that adhere uniformly to the lipid parts of cell membranes. The fluorescent bands are on the outside of the lipid bilayer, which makes them ideal for a variety of uses. They are extensively used for in vitro cell labeling, cell proliferation experiments, and in vivo and in vitro cell tracking studies. Popular models include PKH-67 (green) and PKH-26 (red). Because PKH dyes are extremely sensitive to the lipid bilayers of exosomes, they have excellent staining properties and are widely applied in this field.
- Lipophilic carbocyanine dyes: Fluorescent dyes that can non-covalently attach themselves to the exosome membrane. These dyes emit very faint fluorescence prior to incorporation into the cell wall. Once embedded into the cell wall, dyes can permeate the membrane and label the whole membrane. The common carbocyanine dyes are DiI, DiD, DiO, and DiR. DiR is unique in that it, through its infrared fluorescence, can enter cells and tissues - making it useful for in vivo tracking and imaging.
Permeable dyes
Permeable dyes are capable of penetrating the exosomal membrane and labeling the interior of the exosomes. The commonly used permeable dyes include:
- Carboxyfluorescein diacetate, succinimidyl ester (CFDA): The dye can cross the exosomal barrier, and intracellular esterases convert it into carboxyfluorescein succinimidyl ester (CFSE), which emits green fluorescence. CFSE can then combine with free amines in cytoskeletal proteins to form fluorescent protein complexes.
- Calcein-AM: Calcein-AM diffuses passively to exosomes and is esterased in the exosome to produce stable green fluorescent Calcein, which cannot diffuse out of the exosome. This attribute makes Calcein-AM an excellent choice for exosome labeling and tracking. Moreover, it's less toxic than CFDA, making it an ideal option for live-cell staining.
- C5-maleimide-Alexa 488 / C5-maleimide-Alexa 633: Fluorescent dyes interact with thiol groups on the surface of the exosome through their maleimide moieties and create stable covalent bonds. Such selective labelling makes it possible to mark target molecules in a specific manner on the surface of the exosome.
Near-infrared (NIR) fluorescent probes
This approach uses NIR fluorescent probes that bind to molecules or objects on the exosomal surface and can label and track them. NIR fluorescent probes include organic small molecule dyes, organic nanoparticles containing small molecule dyes, conjugated polymers, quantum dots, rare earth-doped nanoparticles, and single-walled carbon nanotubes. They possess low spontaneous background signals and strong penetration into tissue, making them ideal for in vivo imaging.
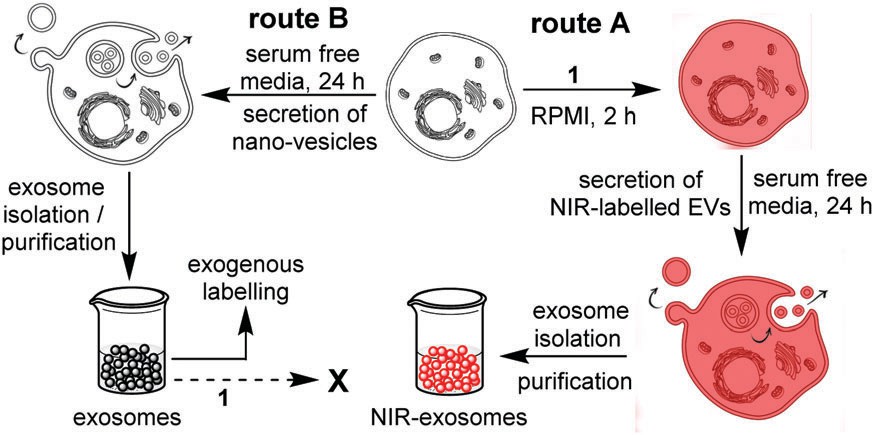 Fig. 2. Strategies for exogenous and endogenous labelling with NIR-AZA (Monopoli, M. P., Zendrini, A., et al., 2018).
Fig. 2. Strategies for exogenous and endogenous labelling with NIR-AZA (Monopoli, M. P., Zendrini, A., et al., 2018).
Physical Labeling Method
Quantum dots (QDs)
Quantum dots are semiconductor nanocrystals that can be used as fluorescent probes to mark biomolecules. They possess an extended excitation range, high quantum yield, and excellent stability, and they are commonly employed for biomedical imaging.
Radioactive labels
When exosomes are labeled with radioactive isotopes, exosomes can be tracked using their radioactive signature. This technique is widely applied in medical imaging procedures, including MRI (magnetic resonance imaging) and CT (computed tomography). The radioactive isotopes are very sensitive and can penetrate well through tissues, typically 89Zr, 99mTc and 131I.
Superparamagnetic iron oxide nanoparticles (SPIONs)
These nanoparticles exhibit superparamagnetism under an external magnetic field and can be used as contrast agents for MRI after binding with exosomes, enabling in vivo imaging.
Gold nanoparticles (GNPs)
GNPs can be combined with exosomes through coating methods such as glucose coating, and subsequent tracking can be performed using CT imaging. GNPs exhibit strong affinity for biomolecules and good biocompatibility.
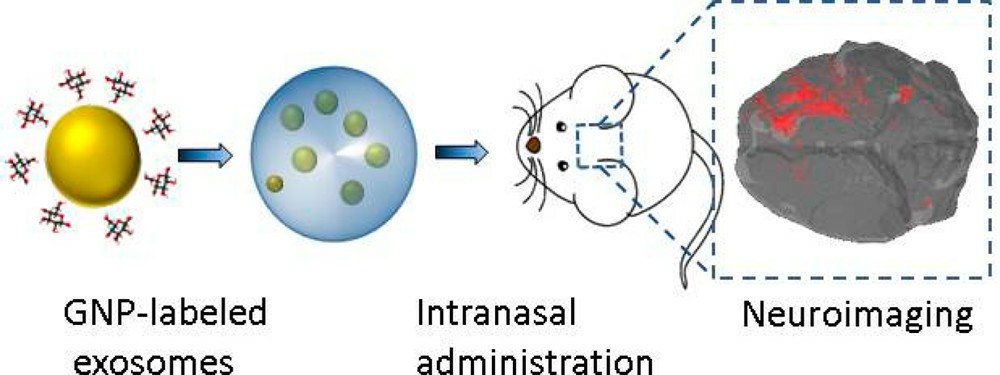 Fig. 3. In vivo neuroimaging of exosomes using gold nanoparticles (Betzer, O., Perets, N., et al., 2017).
Fig. 3. In vivo neuroimaging of exosomes using gold nanoparticles (Betzer, O., Perets, N., et al., 2017).
Biological Methods
Fluorescent protein fusion expression
In molecular biology, fluorescent proteins (e.g., GFP or RFP) can be fused with exosomal marker proteins (e.g., CD63, CD9, CD81) or intracellular molecules. This approach allows us to directly monitor the transit of exosomes from the donor to the target cells. In addition, since fluorescent proteins are co-labelled to exosomes, this labelling approach prevents fake-positives resulting from the labelling of other lipid structures.
Lentiviral-mediated expression
Constructing plasmids encoding specific exosomal proteins (such as CD63) and fluorescent proteins, and then packaging them into lentiviruses to infect cells, can result in exosomes carrying green fluorescence.
Luciferase labeling
By creating plasmids that express luciferase and exosomal marker proteins as fusion proteins and then transfecting cells with those plasmids, you can make exosomes with robust luciferase activity. The most widely used luciferase genes are firefly luciferase (Fluc), gaussia luciferase (Gluc), or renilla luciferase (Rluc). This does not require fluorescence excitation, but instead relies on reactions between the substrate and ATP, Mg2+ or oxygen to create light with high sensitivity and low background.
Other Techniques
Photoacoustic imaging (PAI)
In this approach, contrast molecules within biological samples soak up pulsed laser radiation and transform it into acoustic signals. These signals are then detected and interpreted by a scanning transducer. PAI is a convenient, non-invasive imaging modality offering the combination of ultrasound's deep tissue penetration and spatial resolution with the contrast of an optical image.
Stigmatising exosomes makes it easier to study how exosomes are distributed and stored in the body. It also facilitates drug delivery and release monitoring during therapeutic uses. Therefore, the development of exosome labelling drives biotechnological advancements in associated fields, creating more possibilities for exosome research and applications.
| Products & Services | Description |
| Exosome Applications | Creative Bioarray offers a complete set of services for exosome application including but not limited to exosome transfection, exosome labeling, and exosome targeting. |
| Exosome Analysis | Creative Bioarray provides diverse exosomal species analysis to help you understand your exosome compositions. |
| Exosome Identification | Creative Bioarray provides comprehensive support for your exosome identification by including the morphology assay, purity, and quantity assay, particle size distribution analysis, and exosome-specific markers expression. |
| Exosome Isolation Tools | Creative Bioarray aims to develop the best quality exosome isolation tools with optimized conditions to help our customers obtain pure exosomes with a higher yield. |
References
- Shen LM, et al. Tracking exosomes in vitro and in vivo to elucidate their physiological functions: implications for diagnostic and therapeutic nanocarriers. Acs Applied Nano Materials, 2018, acsanm.8b00601.
- Monopoli, M. P., et al. Endogenous exosome labelling with an amphiphilic NIR-fluorescent probe. Chemical communications (Cambridge, England), 2018, 54(52), 7219–7222.
- Betzer, O., et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS nano, 2017, 11(11), 10883–10893.

