How to Perform Targeted Modification of Exosomes?
Exosomes are an extracellular vesicle (EV) that is widespread in most human tissues. Exploiting exosomes as therapeutics or delivery systems is an ongoing area of focus. But natural exosomes are not particularly capable of targeted attack. It is possible to modify the surface of exosomes, increasing the targeting capacity, and hence increasing the accumulation of exosomes in specific tissues or organs to achieve site-specific drug delivery.
Methods of Exosome Targeting Modification
Gene engineering
The exosome membrane contains proteins called transmembrane and tetraspannin. By genetic engineering, the targeting ligands can be attached to the donor cells' transmembrane proteins, making the exosomes that leak out express the desired molecules or properties. This method can give high-precision control of exosome structure and function, making it a useful tool for individual exosome engineering. But intracellular proteases could break down peptides, thereby lowering the number of active exosomes. Furthermore, targeting components added to the exosome membrane may disrupt the normal functions of proteins on the membrane of the exosome.
- Gene fusion: This method combines the target gene with the exosome membrane's transmembrane protein gene to form a fusion expression plasmid. Then this plasmid is transplanted into donor cells (via plasmid transfection or other processes) and the donor cells release exosomes containing functional molecules on their surface. Targeting peptides, homing peptides and therapeutic proteins are the most commonly employed targeting ligands. Due to their small size, flexibility, biocompatibility, low immunogenicity and targeting properties, peptides are currently the most widely used targeting ligands.
- Gene editing: Tools like CRISPR-Cas9, ZFN and TALEN can be used to modify the genome of exosome-making cells, thereby affecting the genetic structure and activity of the exosomes.
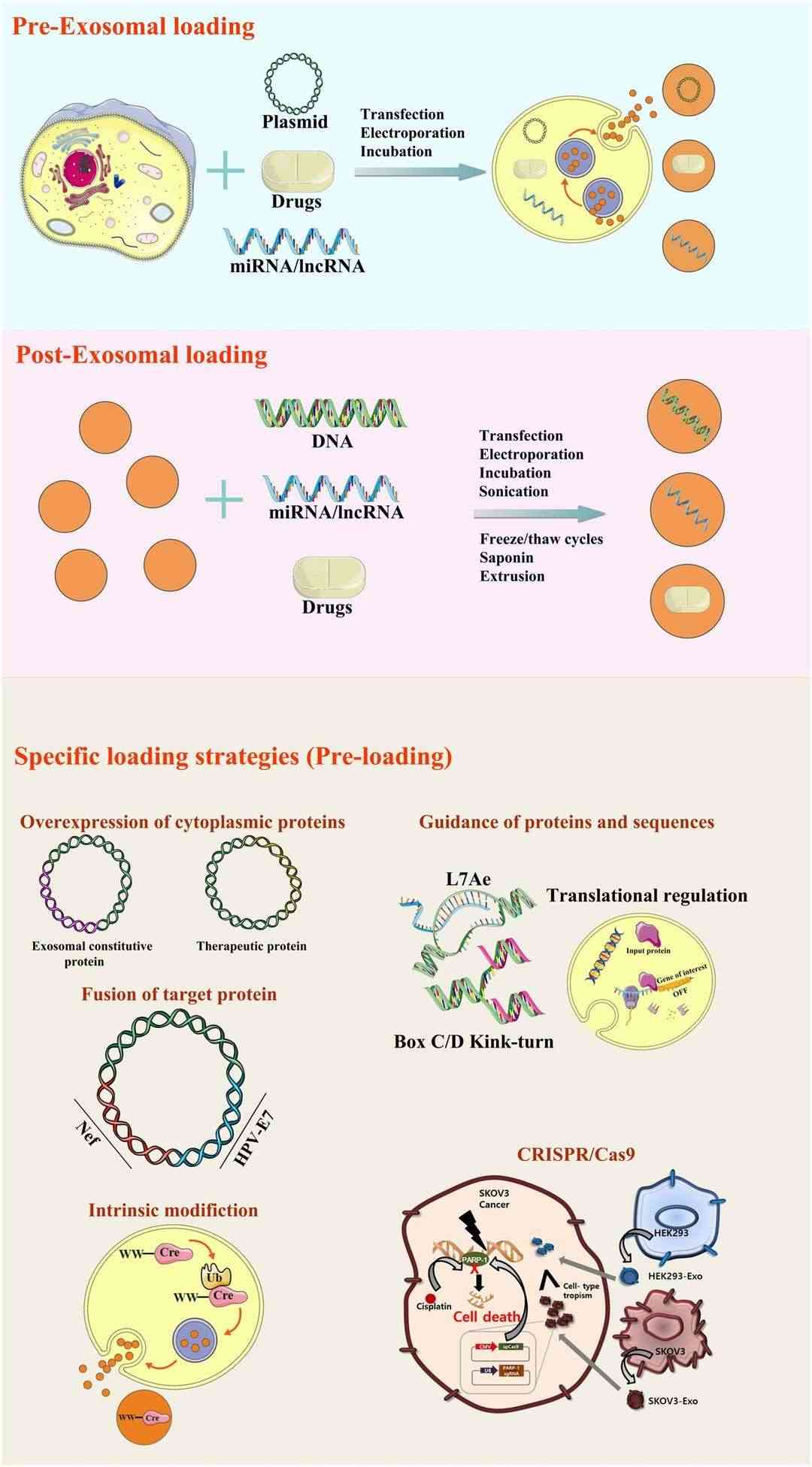 Fig. 1. Design strategies for the modifications of exosomal surface (Xu, M., Yang, Q., et al., 2020).
Fig. 1. Design strategies for the modifications of exosomal surface (Xu, M., Yang, Q., et al., 2020).
Chemical modifications
The chemical modification involves introducing new molecules or groups to the surface or inside of the exosomes through chemical reactions to change their structures and functions. This method is flexible and suitable for various types of modifications.
- Lipid modification: Lipid molecules can be combined with exosomes through liposome fusion or chemical conjugation. Such lipid molecules can affect the exosome's stability, targeting capability and drug-loading capacity.
- Chemical attachment: Peptides, antibodies, proteins, lipids, aptamers, and other molecules are attached to the surface of the exosome by chemical interactions. This approach allows exact control of the types, numbers and positions of altered molecules, allowing multifunctional exosome modifications.
- Click chemistry: Click chemistry-related functional groups (e.g., alkyne and azide groups) can be transferred onto some biomolecules (proteins, lipids, RNA) either on the surface of an exosome or inside it. These motifs can then click reactions with matching functional groups on another molecule (usually probes, drug molecules or functional molecules) to induce targeted changes at specific sites on or inside the exosomes. The benefit of this approach is faster reactions and high purity of the product.
Physical modifications
- Electrostatic interaction: In a reverse-charge attraction approach, the same charge is added to the surface of the exosomal membrane to improve the efficiency of targeting opposite-charged biological membranes.
- Hydrophobic interaction/membrane fusion: Exosomes have a phospholipid bilayer on their surface, which allows them to be hydrophobic with liposomes. These liposome membranes are stocked with peptides, antibodies or polyethylene glycol (PEG), and fused with exosome membranes by freeze-thaw. That produces hybrid exosomes with high-performance targeting without sacrificing the original function of the membrane proteins.
- Magnetic guidance: Exosomes are tagged with magnetic nanoparticles (Fe3O4) and targeted in vivo by an external magnetic field.
- Acoustic guidance: By altering the acoustic waves' frequency and strength, direction of exosome movement can be controlled in liquid media. Acoustic waves can enter biological tissues with minimal cell- and exosome-damage, making acoustic navigation an anti-invasive way to manipulate exosomes.
Considerations
With some key advantages, exosome delivery is the best way to deliver drugs. These include tissue targeting, stability, half-life of the blood, and biocompatibility with low toxicity effects. However, even for these strengths, there are still plenty of hurdles to overcome and optimise for clinical use. One of the big issues is how to make exosomes more targeted. It is possible to overcome these barriers by improving and optimizing the way that we modify. But in practice we must also keep the following in mind.
- The strengths and weaknesses of each technique must be weighed against the experimental goal and conditions in order to select the best option.
- It is essential to measure and confirm whether the process of modification has any effect on exosome biology and function.
- A rigorous quality control and safety evaluation of the reconstituted exosomes is critical for their safety and efficacy in the clinical context.
| Products & Services | Description |
| Exosome Applications | Creative Bioarray offers a complete set of services for exosome application including but not limited to exosome transfection, exosome labeling, and exosome targeting. |
| Exosome Analysis | Creative Bioarray provides diverse exosomal species analysis to help you understand your exosome compositions. |
| Exosome Identification | Creative Bioarray provides comprehensive support for your exosome identification by including the morphology assay, purity, and quantity assay, particle size distribution analysis, and exosome-specific markers expression. |
| Exosome Isolation Tools | Creative Bioarray aims to develop the best quality exosome isolation tools with optimized conditions to help our customers obtain pure exosomes with a higher yield. |
Reference
- Xu, M., et al. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Frontiers in bioengineering and biotechnology, 2020, 8, 586130.

