How to Enhancement Exosome Production?
Exosomes are small, cell-suspended vesicles that have gained attention for diagnostic, drug delivery and therapeutic uses. With the increasing application of exosomes, the demand for its yield is growing, but many challenges still exist for its large-scale production. Standard techniques for separating exosomes from culture media have low yields due to their low secretion rates. On average, less than 1 g of exosomal proteins are typically isolated from each millilitre of culture medium, far from adequate for clinical studies and applications. Additionally, traditional methods like ultracentrifugation not only takes time, but also are inefficient and expensive to scale up. To meet these demands, increasing exosome production productivity and yield is one of the most pressing challenges in our modern world.
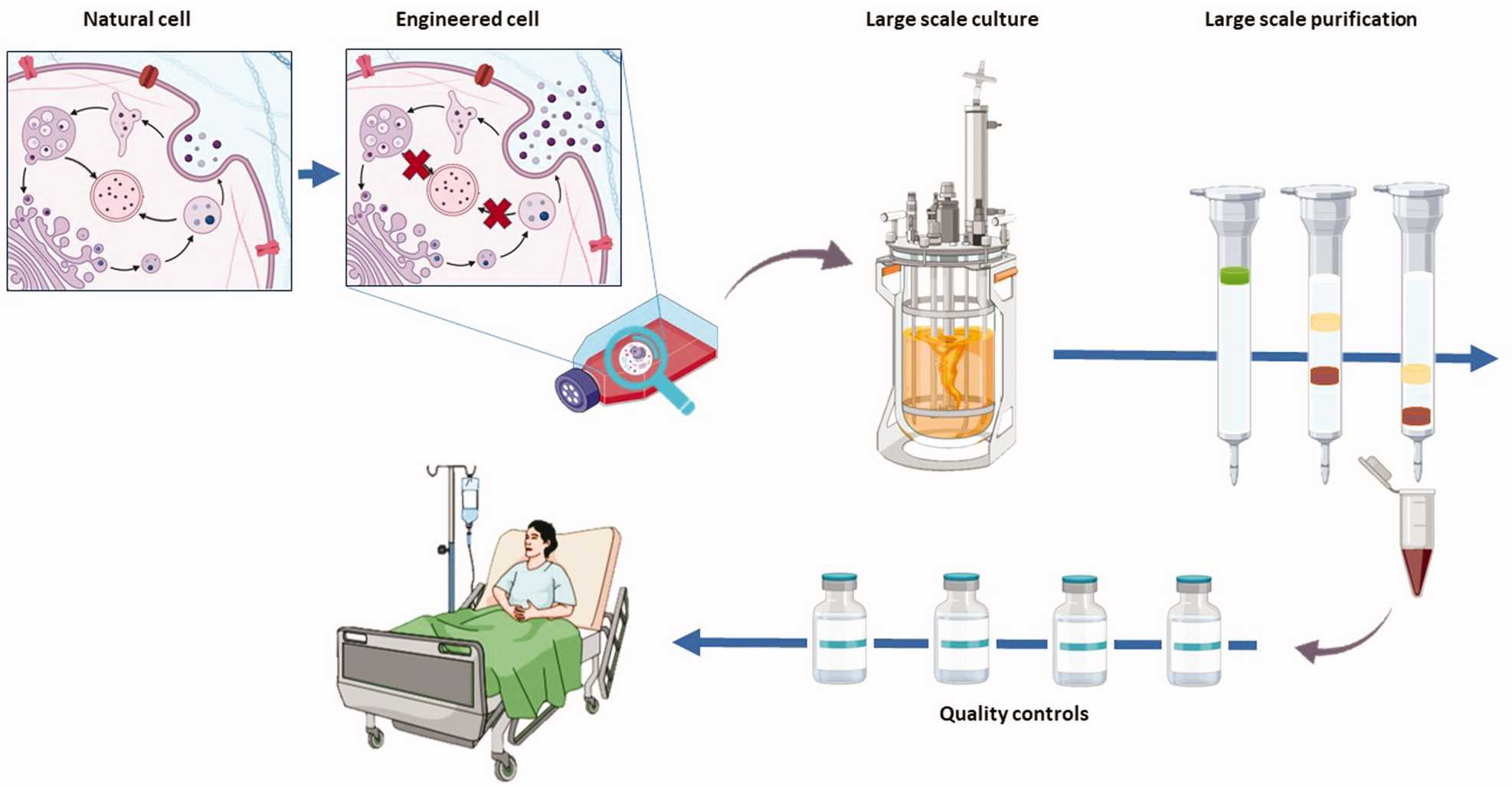 Fig. 1. Schematic diagram of exosome production from the development of engineered cells to bedside application (Jafari D, Malih S., et al., 2020).
Fig. 1. Schematic diagram of exosome production from the development of engineered cells to bedside application (Jafari D, Malih S., et al., 2020).
Methods and Techniques for Expanded Production of Exosomes
Genetic engineering techniques
Scientists can generate exosomes in substantial numbers by knocking down and manipulating genes in the exosome biogenesis and secretion process, including Rab GTPases and elements of the endosomal sorting required transport complex (ESCRT) machinery. Overexpression of genes for membrane dynamics and vesicular transport, for instance, was associated with increased exosome secretion. But the approach also fails to adapt to whatever genetic modification to exosomes might add.
Cell Pretreatment
- Hypoxia: In contrast, hypoxic MSCs secrete more exosomes. Its increased HIF correlates with expression of mediators involved in exosome releasing proteins like ALIX, TSG101, Rab27a and Rab27b. Moreover, the hyperacidic state of the cellular microenvironment created by hypoxia encourages exosome release from tumor cells.
- Cytokines: cytokines, such as IL-1, TNF- and others increased exosome formation and therapeutic activity of the cells. In a neuroinflammatory model, gamma interferon, for instance, improves MSC-EXO's anti-inflammatory activity.
- Thrombin: Thrombin is a serine protease that performs several biological functions. Thrombin pretreatment of human umbilical cord blood mesenchymal stem cells (UCB-MSC) increased EV production at a dose-dependent level.
- Lipocalins: Lipocalins can augment the ceramide content of exosomes by attaching to T-calmodulin and increasing exosomal production.
- Small molecules: Haemoxase inducers, for instance, can increase the release of biologically active exosomes by altering oxidative stress response pathways in parental cells. These are potent, non-toxic enhancers that could significantly ramp up exosome production.
Culture Optimization
- Culture model optimization: Changing from standard two-dimensional (2D) cell cultures to three-dimensional (3D) systems can better mimic in vivo conditions, thereby increasing exosome production and functionality. Specifically, hollow fiber bioreactors and stirred tank bioreactors are the two major technologies currently used for mass-production of exosomes. These reactors allow accurate control of pH, oxygen and nutrient flow within the reactor to maximize cell growth and exosome production. Meanwhile, the exosomes obtained from this 3D culture are more abundant, active, and suitable for more therapeutic applications.
- Culture material optimization: Biomaterials that release certain ions or growth factors may encourage cells to make more exosomes. Substrates that excrete lithium ions, for instance, have been observed to increase exosome angiogenic activity by stimulating specific signalling pathways.
- Medium optimization: Addition of e.g. cytokines (e.g. IFN-γ, IL-1β) to the medium can enhance exosome production by altering cellular metabolism and increasing the expression of exosome-associated proteins. Establishing serum- or chemically defined media can also reduce the amounts of impurities in serum-derived exosomes, thus improving yield and purity.
Cell Source
Cell lines from different sources have a significant impact on the production and secretion of exosomes. For example, MSCs are now being often used as exosome-producing cell lines, and MSCs come from a wide range of sources, such as adipose tissue, umbilical cord, bone marrow, and placenta, whereas placenta-derived MSCs would have greater exosome production than bone marrow-derived MSCs. There are also researchers who tried to generate immortalized cell lines after genetic manipulation of hESC-MSCs with the MYC gene, which can produce exosomes continuously without the need for batch renewal. However, whether the properties of exosomes produced by this approach are affected needs to be further verified.
Aside from these, exosome-producing reactions have been stimulated through small-scale mechanical agitation and high-frequency acoustic stimulation. These strategies make use of physical pressure to release exosomes without disrupting their integrity.
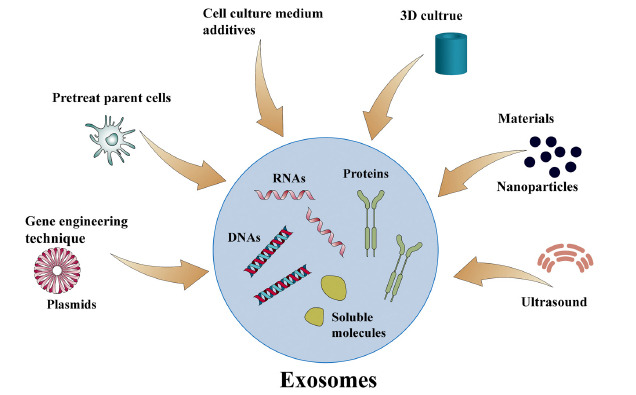 Fig. 2. Approaches of exosome production enhancement (Qu Q, Fu B, et al., 2023).
Fig. 2. Approaches of exosome production enhancement (Qu Q, Fu B, et al., 2023).
The possibility of increasing exosome production has been recently shown to be achievable. Hollow fibre bioreactors for the purification of exosomes from human embryonic kidney cells, for instance, have greatly increased their capacity for research and therapeutic purposes. Similarly, xeno-free media for mesenchymal stem cell cultures can increase exosome production without causing xeno contamination. Hollow fiber bioreactors could double yields by 5-10 folds when exosomes were prepared from HEK293 cells.
| Products & Services | Description |
| Exosome Identification | Creative Bioarray provides comprehensive support for your exosome identification by including the morphology assay, purity, and quantity assay, particle size distribution analysis, and exosome-specific markers expression. |
| Exosome Isolation Tools | Creative Bioarray aims to develop the best quality exosome isolation tools with optimized conditions to help our customers obtain pure exosomes with a higher yield. |
References
- Qu Q, Fu B, et al. Current Strategies for Promoting the Large-scale Production of Exosomes. Curr Neuropharmacol. 2023. 21(9):1964-1979.
- Jafari D, Malih S, et al. Gholipourmalekabadi M, Sadeghizadeh M, Samadikuchaksaraei A. Improvement, scaling-up, and downstream analysis of exosome production. Crit Rev Biotechnol. 2020. 40(8):1098-1112.
- Kimiz-Gebologlu I, Oncel SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022. 347:533-543.

