- HCC-78
- HDLM-2
- DOHH-2
- L-540
- MX-1
- NALM-6
- NB-4
- CAL-51
- SNB-19
- KYSE-520
- MKN-45
- BA/F3
- MS-5
- HCEC-B4G12
- NK-92
- PA-TU-8988S
- MONO-MAC-1
- PA-TU-8902
- Human Microglia
- Human Hepatic Stellate Cells
- Human Skeletal Muscle Cells (DMD)
- Human Schwann Cells
- Human Oral Keratinocytes (HOK)
- Human Cardiomyocytes
- Human Small Intestinal Epithelial Cells
- Human Colonic Epithelial Cells
- Human Intestinal Fibroblasts
- Primary Human Large Intestine Microvascular Endothelial Cells
- Human Small Intestinal Microvascular Endothelial Cells
- Human Retinal Pigment Epithelial Cells
- Human Hepatocytes
- Cynomolgus Monkey Lung Microvascular Endothelial Cells
- Cynomolgus Monkey Vein Endothelial Cells
- C57BL/6 Mouse Primary Mammary Epithelial Cells
- C57BL/6 Mouse Vein Endothelial Cells
- Rat Primary Kidney Epithelial Cells
- Rat Gingival Epithelial Cells
- Rabbit Lung Endothelial Cells
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY

Human Epidermal Keratinocytes-adult (HEK-a)
Cat.No.: CSC-7791W
Species: Human
Source: Epidermis
Cell Type: Keratinocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human epidermal keratinocytes-adult (HEK-a) constitute over 90% of the epidermis, forming the skin's physical barrier and playing a crucial role in innate immune defense. The keratinized stratified squamous epithelium is composed of keratinocytes. These cells undergo a series of differentiation or keratinization processes within the epidermis, transitioning from the basal layer upwards into the spinous, granular, and cornified layers. The morphology and function of keratinocytes in each layer are governed by complex and tightly regulated mechanisms. Transcription factors such as p63 and AP-2γ, as well as the Notch and Wnt signaling pathways, play crucial roles in regulating the proliferation, differentiation, and survival of keratinocytes.
These cells can identify various cytokines and pathogen-associated molecular patterns. By expressing multiple pro-inflammatory cytokines and chemokines, as well as cytokine receptors and microbial sensors like Toll-like receptors (TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR9), MDA5 (melanoma differentiation-associated gene 5), and RIG-I (retinoic acid-inducible gene I), HEK facilitate the recruitment of inflammatory cells. Simultaneously, by expressing antimicrobial peptides such as defensins, cathelicidins, and S100 proteins, HEK display antimicrobial activity. Through these immune functions, HEK significantly contribute to the pathogenesis of inflammatory skin disorders such as atopic dermatitis (AD), psoriasis, and pustular skin diseases. Thus, HEK-a are often employed to simulate the onset and progression of skin diseases, aiding in the in-depth exploration of pathogenic mechanisms and treatment strategies. Additionally, as signs of aging gradually appear in the skin with advancing age, HEK-a are frequently used to screen anti-aging drugs to evaluate their effect on improving skin aging.
 Fig. 1. Primary culture of normal human epidermal keratinocytes (Barthe M ,Jean-Paul Thénot, et al., 2020).
Fig. 1. Primary culture of normal human epidermal keratinocytes (Barthe M ,Jean-Paul Thénot, et al., 2020).
TRPV3 Stimulation Suppresses Proliferation of Epidermal Keratinocytes and Induces Cell Death
The functions of TRP channels were initially focused on sensory neurons, primarily linked to sensory functions such as pain, itch, thermal hyperalgesia, thermosensation, and mechanosensation. Recent decades have seen research indicating the presence of TRP channels in non-neuronal cells, especially their functions in the skin. As part of the TRP channel family, TRPV3 was first discovered in keratinocytes. Studies have shown that TRPV3 affects skin health, including epidermal barrier formation, hair growth, and melanogenesis. Szöllősi et al. further investigated the native role of TRPV3 in normal human epidermal keratinocytes (NHEKs) to elucidate its effect on the most abundant skin cell type at the cellular level.
They explored the cellular effects of TRPV3 activation on the growth and survival of NHEKs by exposing them to various TRPV3 agonists, such as carvacrol (a plant derivative) and the synthetic agonist 2-APB. Results showed that carvacrol and 2-APB dose-dependently decreased cell viability and proliferation of NHEK cells (Fig. 1a and b). TRPV3 specificity of carvacrol and 2-APB actions was further assessed and validated through RNAi. Silencing TRPV3 almost completely prevented the negative impact on cell viability and proliferation, demonstrating TRPV3's crucial role in these cellular processes. High concentrations of carvacrol and 2-APB significantly reduced mitochondrial membrane potential (an early apoptotic marker) without affecting membrane integrity, indicating apoptotic cell death (Fig. 2a). The calcium chelator EDTA had no impact on these results, suggesting the effect is independent of calcium mobilization from the extracellular space (Fig. 2b). The above results indicate that TRPV3 stimulation inhibits human epidermal keratinocyte proliferation and induces cell death.
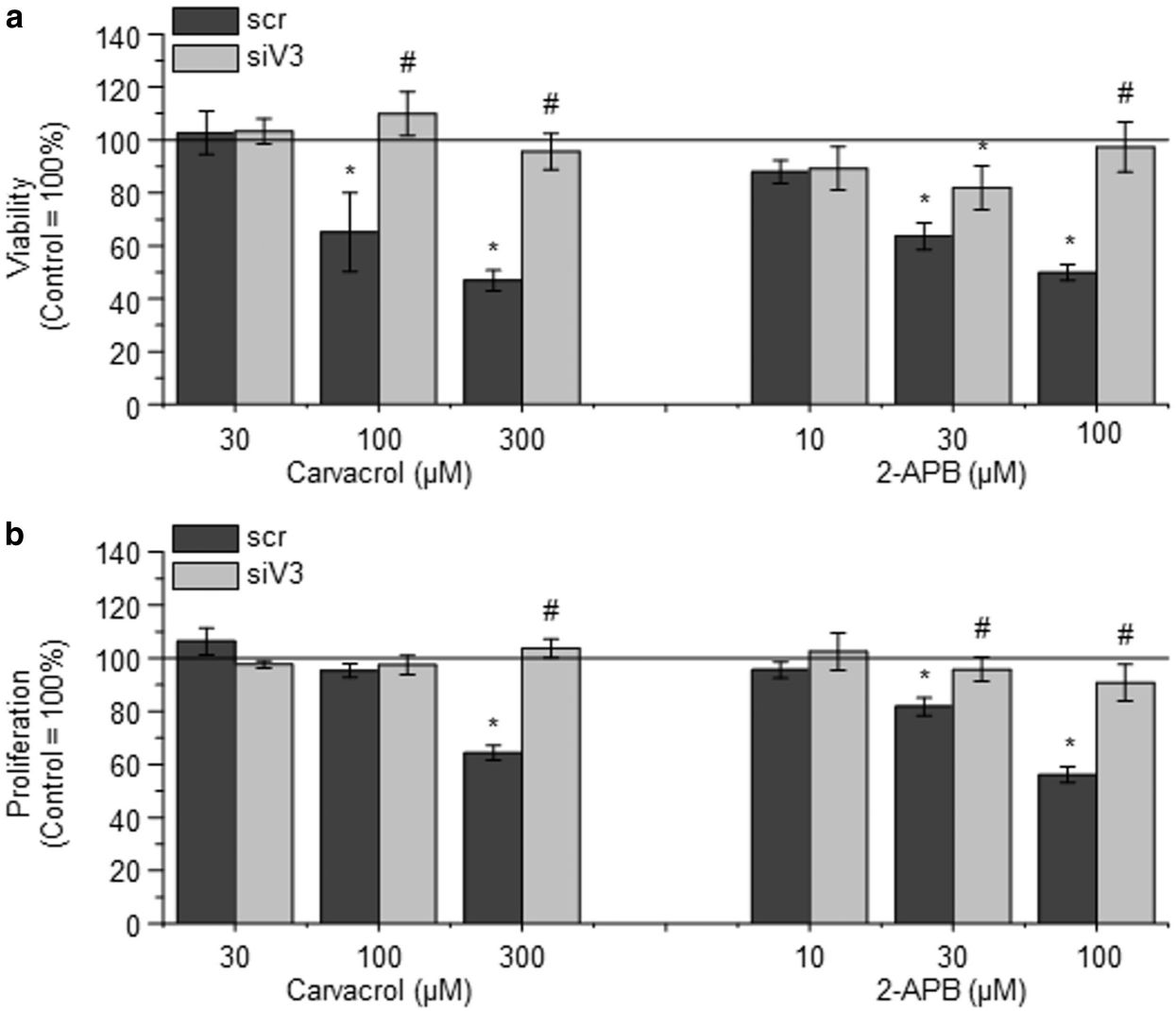 Fig. 1. Activation of TRPV3 on normal human epidermal keratinocytes (NHEKs) decreases viability and inhibits proliferation (Szöllősi AG, Vasas N, et al., 2018).
Fig. 1. Activation of TRPV3 on normal human epidermal keratinocytes (NHEKs) decreases viability and inhibits proliferation (Szöllősi AG, Vasas N, et al., 2018).
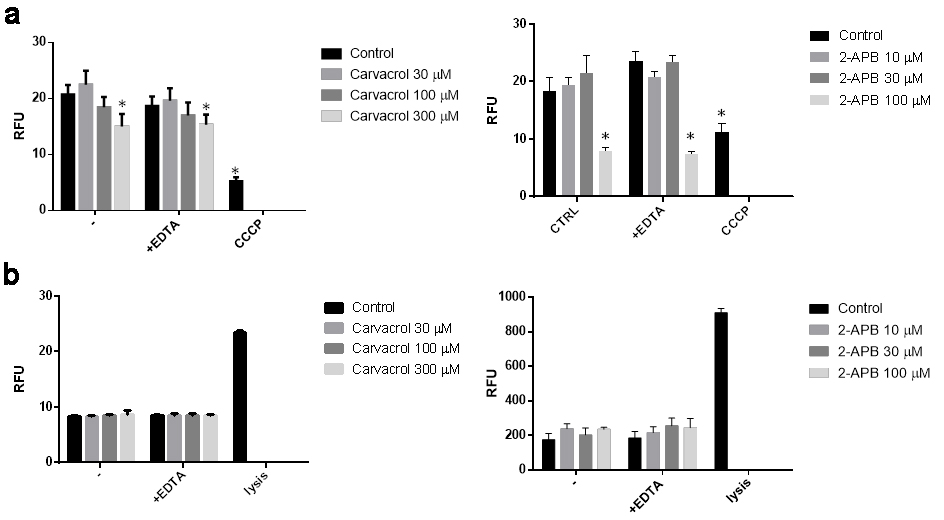 Fig. 2. Activation of transient receptor potential vanilloid-3 (TRPV3) on normal human epidermal keratinocytes (NHEK) causes apoptosis (a) and does not cause necrosis (b) (Szöllősi AG, Vasas N, et al., 2018).
Fig. 2. Activation of transient receptor potential vanilloid-3 (TRPV3) on normal human epidermal keratinocytes (NHEK) causes apoptosis (a) and does not cause necrosis (b) (Szöllősi AG, Vasas N, et al., 2018).
6-Shogaol Prevents UVB Radiation Mediated Inflammation and Oxidative Stress in Human Epidermal Keratinocytes
The skin can resist environmental damage, but excessive ultraviolet (UV) radiation increases the risk of inflammation, photoaging, and nonmelanoma skin cancers. UVB radiation elevates reactive oxygen species (ROS), leading to oxidative stress. Nuclear factor erythroid 2-related factor 2 (Nrf2) plays a crucial role in combating oxidative stress. 6-Shogaol, derived from ginger, has demonstrated anti-inflammatory and antioxidative properties. While it activates Nrf2 signaling in HEK293 and HepG2 cells, its function in human epidermal keratinocytes is still unclear. Chen's team investigated the mechanisms underlying 6-shogaol's pharmacological effects by assessing ROS, Nrf2, and MAPK signaling pathways.
Measurements of ROS levels and inflammation markers (COX-2 and iNOS) showed that 6-shogaol significantly inhibited ROS production and the expression of COX-2 and iNOS, suggesting it suppresses UVB-induced inflammation in keratinocytes. UVB radiation generates ROS, activating MAPK pathways and causing oxidative stress. To clarify the molecular mechanisms, they examined the MAPK family in UVB-irradiated keratinocytes. The results showed that UVB increased p38, ERK, and JNK levels (Fig. 3A and B). In contrast, 20 μg of 6-shogaol significantly reduced their phosphorylation compared to UVB-only cells. Western blot analysis confirmed that 6-shogaol inhibits UVB-induced overexpression of these proteins (Fig. 3D). Additionally, UVB exposure decreased Nrf2 levels, but 6-shogaol prevented this reduction. UVB (180 mJ/cm²) significantly lowered Nrf2 protein levels, while 6-shogaol preserved them and increased HO-1 levels in UVB-irradiated cells (Fig. 4A). RT-PCR results for Nrf2 and HO-1 mRNA expression confirmed these findings (Fig. 4B). Overall, 6-shogaol may serve as an effective agent to enhance resistance against UVB-induced inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes.
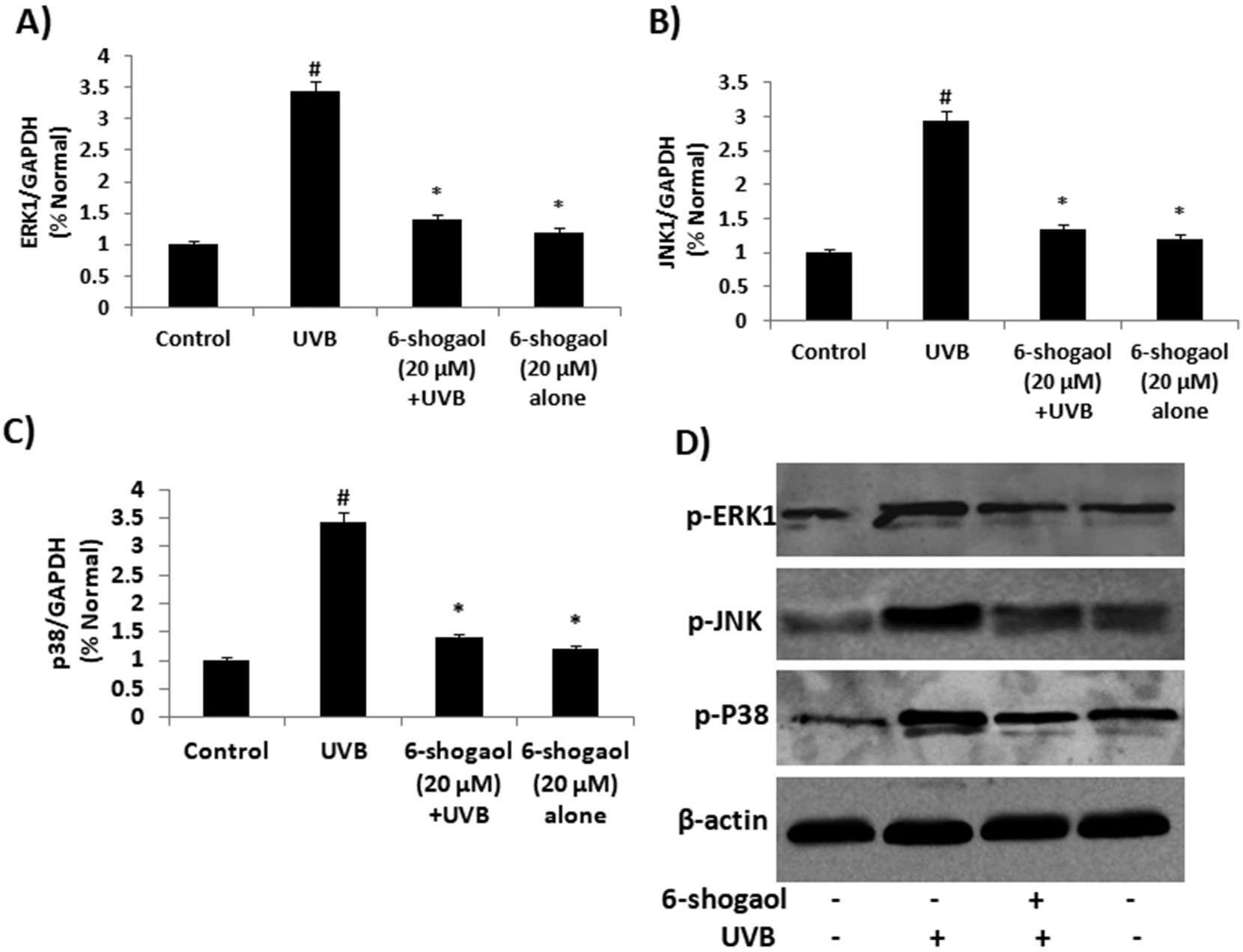 Fig. 3. 6-shogaol on UVB induced MAPKs signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
Fig. 3. 6-shogaol on UVB induced MAPKs signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
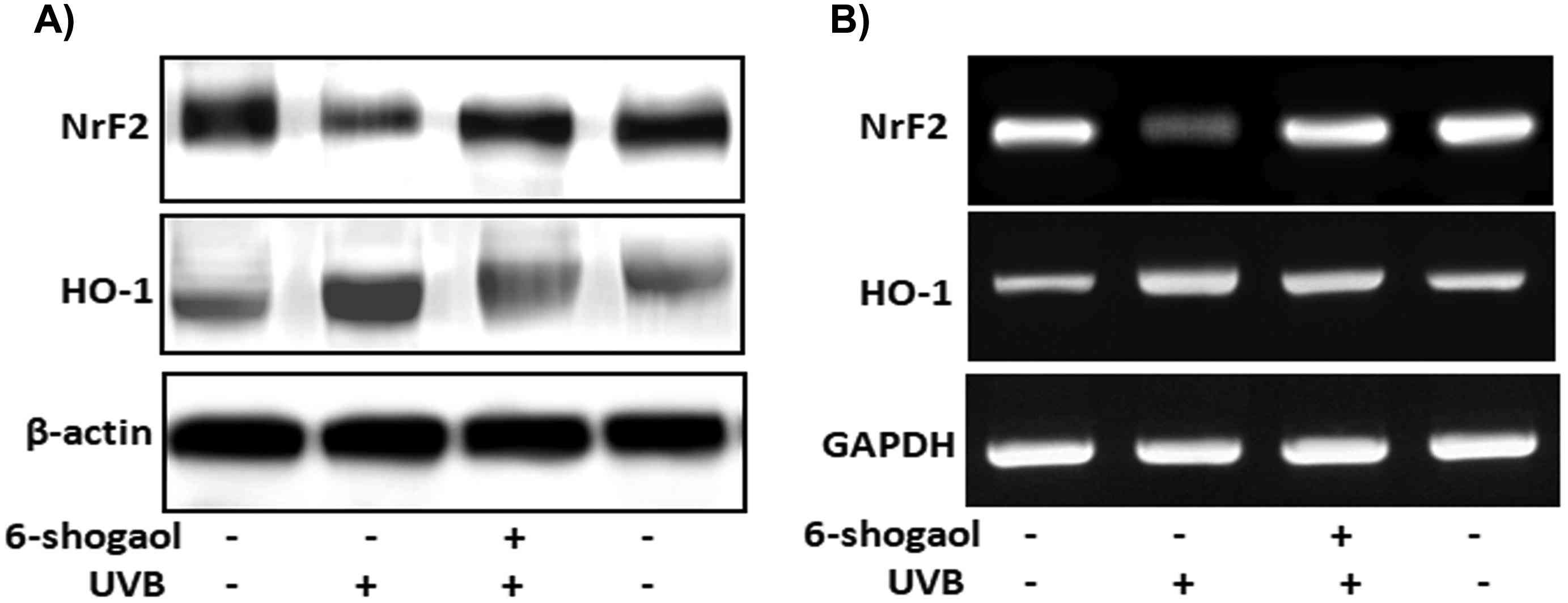 Fig. 4. 6-shogaol on UVB induced NrF2 signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
Fig. 4. 6-shogaol on UVB induced NrF2 signaling in human epidermal keratinocytes (Chen F, Tang Y, et al., 2019).
Ask a Question
Write your own review