Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
HCEC-B4G12
Cat.No.: CSC-C3457
Species: Human
Source: corneal endothelium
Morphology: cuboid epitheloid cells growing strongly adherent in monolayers
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Immunology: cytokeratin +, cytokeratin-7 +, cytokeratin-8 (+), cytokeratin-17 -, cytokeratin-18 +, cytokeratin-19 -, desmin -, endothel -, EpCAM -, GFAP -, neurofilament -, vimentin +
Viruses: P
The HCEC-B4G12 cell line originates from human corneal endothelial cells and has undergone genetic modification to support long-term culture while preserving corneal endothelial cell traits for extended cultivation. This cell line displays polygonal cells that maintain tight packing patterns which mimic natural corneal endothelial cells. The HCEC-B4G12 cell line expresses key corneal endothelial cell markers such as ZO-1, occludin, connexin-43 together with the corneal endothelium 9.3 E antigen (HCEC9.3 E).
Research demonstrates that the HCEC-B4G12 cell line displays similarities in both characteristics and functional capabilities to mature corneal endothelial cells found in vivo. The HCEC-B4G12 cell line functions as an excellent model for research on corneal endothelial cell activities and disease pathophysiology. Basic biological research can utilize this cell line to examine the physiological functions and cellular metabolism of corneal endothelial cells as well as their other properties. Ophthalmic disease research can use the HCEC-B4G12 cell line to study the development of conditions like glaucoma and corneal endothelial dystrophy and to test drug effectiveness. Moreover, this cell line can be used in tissue engineering research to attempt building corneal endothelial tissue, providing new methods and strategies for the treatment of corneal diseases.
Complexed Ubiquinol Lowers ROS in HCEC-B4G12 and is not Toxtic to HCEC-B4G12
Donor tissues, such as those for corneal transplantation, face significant challenges during hypothermic preservation, including defective cellular metabolism, impaired mitochondrial function, and increased cell death. These issues mainly result from oxidative stress due to accumulated reactive oxygen species (ROS) within cells. Naguib's team developed a novel and stable formulation combining ubiquinol with gamma cyclodextrin (γ-CD) to enhance ubiquinol's aqueous stability and antioxidant activity.
This complex was tested for its ability to scavenge intracellular ROS and toxicity using a high-fidelity immortalized human endothelial cell culture model (HCEC-B4G12). ROS levels were significantly lower in B4G12 cells when complexed ubiquinol at low and high concentrations (1 μM and 100 μM) were used, compared to free ubiquinol in suspension (Fig. 1A and 1B). γ-CD alone was not active. The complex at low concentration (1 μM) was more efficient than ubiquinol at high concentration (100 μM) in ROS mitigation in B4G12 cells. The complex at an equivalent ubiquinol concentration of 100 μM showed complete peak shift by an order of magnitude, and the fluorescence (geometric mean) of cells in this group was about one tenth of that of cells treated with 100 μM of free ubiquinol, indicating total abolition of ROS levels in corneal endothelial cells at this concentration. MTS assay results using HCEC-B4G12 cells tested after 24 and 72 h of exposure showed no inhibition of proliferation or signs of cell death for complexed ubiquinol, free ubiquinol, or γ-CD at all concentrations tested (Fig. 1C). No microscopic signs indicating signs of cell stress or death after 72 h of treatment groups were detected for all groups.
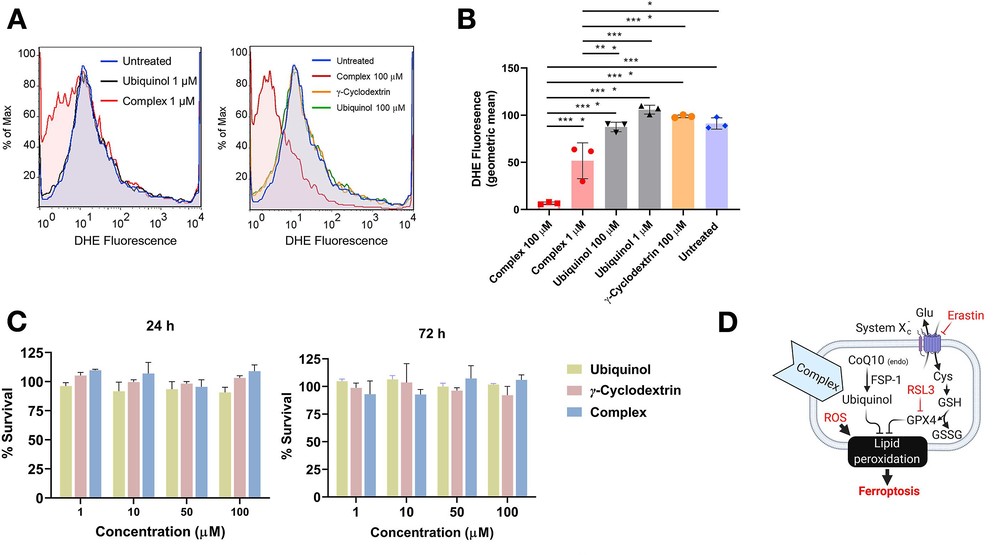 Fig. 1. (A-B) Complexed ubiquinol lowers ROS in human corneal endothelial cells (HCEC-B4G12). (C) Complexed ubiquinol is not toxic to human corneal endothelial cells (Naguib Y W, Saha S, et al., 2021).
Fig. 1. (A-B) Complexed ubiquinol lowers ROS in human corneal endothelial cells (HCEC-B4G12). (C) Complexed ubiquinol is not toxic to human corneal endothelial cells (Naguib Y W, Saha S, et al., 2021).
UV Exposure Activates TRPV Channels and Contributes to the Endothelial Pumping Dysfunction
Fuchs endothelial corneal dystrophy (FECD) is caused by ultraviolet A (UVA) exposure, which damages the corneal endothelium's barrier and pump functions by releasing reactive oxygen species (ROS). The link between UVA exposure levels and TRPV ion channel activation hasn't been explored. Kim's team examined how UVA exposure affects TRPV1 and TRPV4 activation in corneal cells, influencing cell size and junction proteins, to understand their role in the disease's progression.
HCEC-B4G12 was cultured for 7 days from the initial seeding density at 1x104 cells/cm2 for creating monolayered cornea endothelium before applying UVA. Using an UVA light source (365 nm, 2.4mW/cm2), they irradiated UVA daily for 4 weeks to the cells with 2 J/cm2 (4 min), 4 J/cm2 (8 min), and 8 J/cm2 (16 min) energy levels based on the damage threshold of cornea endothelium to simulate chronic FECD. During the experiments, the cell morphology, size, junction protein, and TRPV 1 and 4 were observed. For quantification of these images, MATLAB and image J were utilized for accurate data analysis. Before exposing UVA, they confirmed the expression of ZO-1 and hexagonal morphology of the corneal endothelium. As increasing UVA intensity, cell enlargement and shape distortion from initial hexagonal shape have been observed without significant alteration of ZO-1 expression. Compared to each group, the most substantial size alterations were observed from the case of the 8 J/cm2. For TRPV 1 and 4 expression studies, they were able to confirm that UVA showed agonistic effect for both TRPV channels. There was no TRPV1 expression for intact corneal endothelial cells, whereas UVA treatment induced TRPV1 expression with the UVA exposure level. For TRPV4 expression, they observed the expression on intact corneal endothelial cells and the level of TRPV4 expression increased as UVA exposure level increased (Fig. 2).
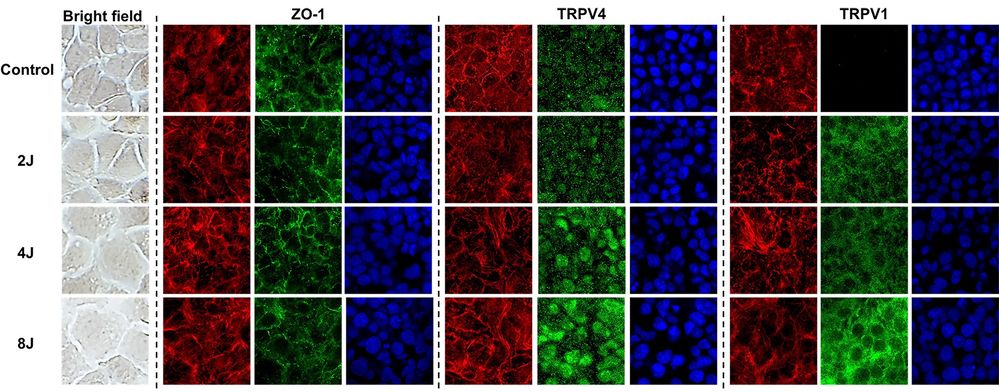 Fig. 2. The effect of ultraviolet A on TRPV1 and 4 activations on corneal endothelium (Kim J; Kim M, et al., 2023).
Fig. 2. The effect of ultraviolet A on TRPV1 and 4 activations on corneal endothelium (Kim J; Kim M, et al., 2023).
Metrics such as cell viability, confluency, passage number, and contamination rates are routinely monitored to establish benchmarks for quality control in cell biology experiments.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Promising results
We were able to use this tumor cell product to test the efficacy of various cancer treatments, with promising results.
29 Mar 2023
Ease of use
After sales services
Value for money
Write your own review