Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Thyroid Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
The thyroid gland, a master regulator of metabolism, plays a pivotal role in maintaining physiological homeostasis. However, the transformation of thyroid cells into tumor cells poses a significant health concern. The thyroid gland consists of follicular and parafollicular cells, each with distinct functions. Follicular cells are responsible for thyroid hormone synthesis, while parafollicular cells, also known as C cells, secrete calcitonin. The transformation of these cells into tumor cells is a multifaceted process involving genetic mutations, epigenetic alterations, and environmental factors.
The Biology of Thyroid Tumor Cells
- Genetic alterations. Mutations in key genes such as BRAF, RAS, and RET are implicated in the pathogenesis of thyroid cancer. These mutations disrupt normal cellular signaling pathways, leading to uncontrolled proliferation and tumor formation. For instance, the BRAF V600E mutation is commonly associated with papillary thyroid cancer, the most prevalent thyroid cancer subtype.
- Epigenetic changes. Epigenetic modifications, including DNA methylation and histone modification, also contribute to thyroid tumor development. These changes can silence tumor suppressor genes or activate oncogenes, fostering an environment conducive to cancer growth.
- Tumor heterogeneity. Thyroid tumors exhibit significant heterogeneity, both at the genetic and histopathological levels. This diversity is reflected in the range of thyroid cancer types, including papillary, follicular, medullary, and anaplastic thyroid cancers. Each type presents unique challenges in terms of diagnosis and treatment, necessitating a tailored approach to patient management.
Molecular Testing
Molecular testing of thyroid tumor cells has revolutionized the diagnostic process. Techniques such as next-generation sequencing (NGS) allow for the detection of specific mutations, aiding in the diagnosis and prognosis of thyroid cancer. For example, the presence of the BRAF V600E mutation can indicate a more aggressive tumor phenotype.
Therapeutic Development
Thyroid tumor cells serve as essential models for developing targeted therapies. The unique genetic profile of these cells provides opportunities for precision medicine, where treatments are tailored to the individual's genetic makeup. For example, tyrosine kinase inhibitors (TKIs) such as sorafenib and lenvatinib have been approved for advanced thyroid cancers, demonstrating significant improvements in progression-free survival. These agents target key signaling pathways involved in tumor growth and angiogenesis, offering a more personalized approach to treatment.
Model for Cancer Research
Thyroid tumor cells provide a valuable model for studying fundamental aspects of cancer biology. Researchers use these cells to investigate processes such as angiogenesis, metastasis, and tumor immune evasion. Insights gained from these studies contribute to a broader understanding of cancer and may lead to the development of new therapeutic strategies applicable to various cancer types.
NRXN2 Suppressed THCA Cell Proliferation and Metastasis
Thyroid cancer (THCA) is a common endocrine malignant tumor, and the global incidence of THCA has increased significantly. Neurexin 2 (NRXN2) is involved in the progression of some diseases. Fig. 1a presents that, in comparison with adjacent normal tissues, the expression of NRXN2 mRNA was largely reduced in 35 paired tissues of THCA patients (P < 0.05). Besides, Fig. 1b demonstrates a decreased expression of NRXN2 mRNA in CAL-62, BCPAP, TPC-1, and 8505C THCA cell lines, compared to that in human normal thyroid epithelial cell line (Nthy-ori 3-1) (P < 0.05). Our results implied that NRXN2 was probably implicated in THCA initiation and development. The CCK-8 assay was exploited to evaluate the impacts of NRXN2 on the proliferation of NRXN2-overexpressed THCA cells. OVER-NRXN2 expression cassettes were designed for overexpressing NRXN2. Our data exposed that OVER-NRXN2 had a high overexpression efficiency. NRXN2 was overexpressed by transfection with OVER-NRXN2 in CAL-62 and TPC-1 cells (P < 0.001, Fig. 1c, d). Overexpressing NRXN2 greatly retarded THCA cell proliferation (P < 0.05, Fig. 1e, f).
The Transwell assay was performed to verify if NRXN2 could regulate THCA cell migration and invasion in vitro. After overexpressing NRXN2 in CAL-62 and TPC1 cells, cell migration and invasion assays were conducted. The result showed that the number of adhered cells on the lower chamber membrane surface was significantly decreased compared to the control group (Fig. 2a-h). These results indicated that NRXN2 could suppress THCA cell metastasis.
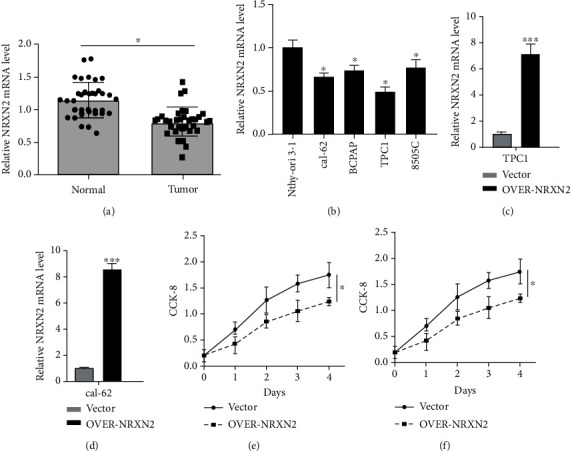 Fig. 1 NRXN2 expression was downregulated in THCA. (a) Differences in NRXN2 expression between 35 THCA tissues and matched adjacent tissues. (b) Differences in NRXN2 expression between 4 different THCA cell lines (CAL-62, BCPAP, TPC-1, and 8505C) and human thyroid cell lines (Nthy-ori 3-1). (c, d) Determination of NRXN2 expression level in OVER-NRXN2 transfected TPC1 cell line and OVER-NRXN2 transfected CAL-62 cell line. (e, f) CCK-8 analysis of the influence of NRXN2 overexpression on TPC1 cell proliferation, and CAL-62 cell proliferation. (Ma C, et al, 2021)
Fig. 1 NRXN2 expression was downregulated in THCA. (a) Differences in NRXN2 expression between 35 THCA tissues and matched adjacent tissues. (b) Differences in NRXN2 expression between 4 different THCA cell lines (CAL-62, BCPAP, TPC-1, and 8505C) and human thyroid cell lines (Nthy-ori 3-1). (c, d) Determination of NRXN2 expression level in OVER-NRXN2 transfected TPC1 cell line and OVER-NRXN2 transfected CAL-62 cell line. (e, f) CCK-8 analysis of the influence of NRXN2 overexpression on TPC1 cell proliferation, and CAL-62 cell proliferation. (Ma C, et al, 2021)
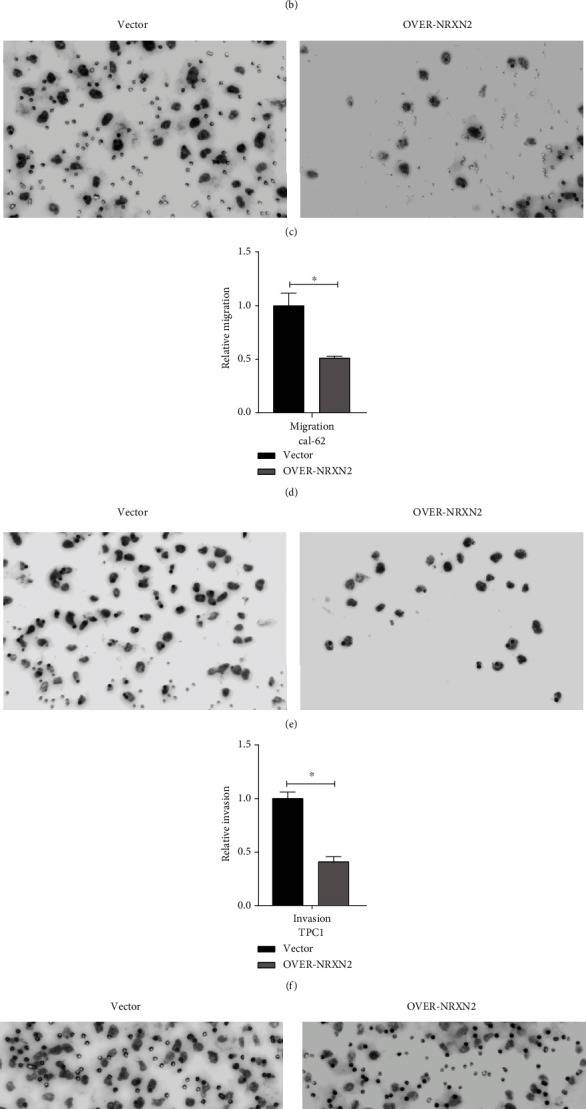 Fig. 2 NRXN2 decreased cell metastasis in THCA cells. (a, b) Transwell assay analysis of the impacts of NRXN2 overexpression on TPC1 cell invasion. (c, d) Transwell assay analysis of the impacts of NRXN2 overexpression on TPC1 cell migration. (e, f) Transwell assay analysis of the impacts of NRXN2 overexpression on CAL-62 cell invasion. (g, h) Transwell assay analysis of the impacts of NRXN2 overexpression on CAL-62 cell migration. (Ma C, et al, 2021)
Fig. 2 NRXN2 decreased cell metastasis in THCA cells. (a, b) Transwell assay analysis of the impacts of NRXN2 overexpression on TPC1 cell invasion. (c, d) Transwell assay analysis of the impacts of NRXN2 overexpression on TPC1 cell migration. (e, f) Transwell assay analysis of the impacts of NRXN2 overexpression on CAL-62 cell invasion. (g, h) Transwell assay analysis of the impacts of NRXN2 overexpression on CAL-62 cell migration. (Ma C, et al, 2021)
Expression of Stc1 in Normal Thyroid and Thyroid Tumor Cells
Stanniocalcin (STC) is a secreted glycoprotein known to regulate serum calcium and phosphate homeostasis. Two STCs, STC1 and STC2, are present in fish and mammals and are expressed in a wide variety of tissues, including the heart, lung, liver, adrenal gland, kidney, prostate, and ovary for STC1, and pancreas, spleen, kidney, and skeletal muscle for STC2. In recent years, the relationship of STC expression to cancer was described in various tissues including the colon, breast, ovary, liver, and esophagus. The higher expression levels of STC1 and STC2 are generally correlated with poor prognostic outcomes of cancers.
STC1 expression was examined in four human FTC-derived cell lines, FTC133, FTC236, FTC238, and RO82-W-1, and one anaplastic thyroid carcinoma-derived cell line, 8305C, by qRT-PCR (Fig. 3A). No mouse thyroid cancer-derived cell lines were available. The three human FTC lines were derived from the same patient; the higher the number, the more distant the metastasis was, which can be considered less differentiated. The expression levels of STC1 mRNA increased as the number became larger in FTC lines of cells, while STC1 expression was lower in RO82-W-1 derived from the metastasis of differentiated follicular carcinoma cells than FTC236, and 8305C obtained from the undifferentiated thyroid carcinoma, had similar STC1 expression level to FTC236. Levels of STC1 mRNA expression in FTC236, FTC238, RO82-W-1, and 8305C were all significantly higher than that of FTC133. STC1 mRNA expression of normal human thyroid was obtained at similar levels to that of RO82-W-1. Western blotting demonstrated the highest protein expression found for FTC238 cells (Fig. 3B). Thus, it appears that both mRNA and protein expression levels for STC1 are roughly correlated with each other and that the expression is roughly inversely correlated with the differentiation status of cells.
Immunohistochemistry was carried out to determine the pattern of STC1 expression in mouse and human thyroids. The results showed that the STC1 antigen was weakly detected in nuclei of many normal mouse thyroid cells with occasional strong expression (Fig. 4A). Focal strong STC1 staining was observed mainly in the cytoplasm of mouse thyroid adenoma tissues (Fig. 4B). Normal human thyroid follicular epithelium was often, but not always, immunoreactive in the nucleus (Fig. 4C). Some human thyroid carcinomas and a thyroid carcinoma cell line had focal strong STC1 immunoreactivity in the nucleus (Fig. 4D-F).
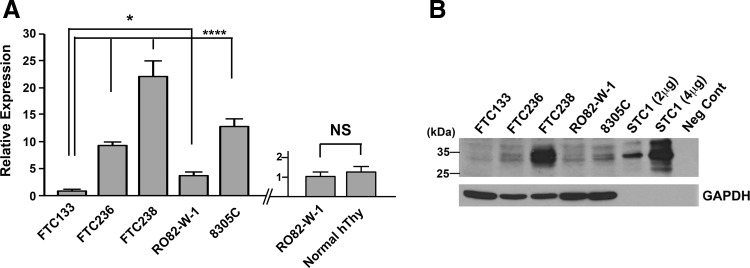 Fig. 3 Analysis of STC1 expression in thyroid cancers. (A) qRT-PCR analysis of STC1 expression in human thyroid carcinoma derived FTC133, FTC236, FTC238, RO82-W-1, and 8305C cells (left). qRT-PCR analysis of STC1 expression in the RO82-W-1 thyroid carcinoma cell line and normal human thyroid tissue (right). (B) Representative Western blotting of STC1 protein expression using a whole cell lysate of the aforementioned cells. (Hayase S, et al., 2015)
Fig. 3 Analysis of STC1 expression in thyroid cancers. (A) qRT-PCR analysis of STC1 expression in human thyroid carcinoma derived FTC133, FTC236, FTC238, RO82-W-1, and 8305C cells (left). qRT-PCR analysis of STC1 expression in the RO82-W-1 thyroid carcinoma cell line and normal human thyroid tissue (right). (B) Representative Western blotting of STC1 protein expression using a whole cell lysate of the aforementioned cells. (Hayase S, et al., 2015)
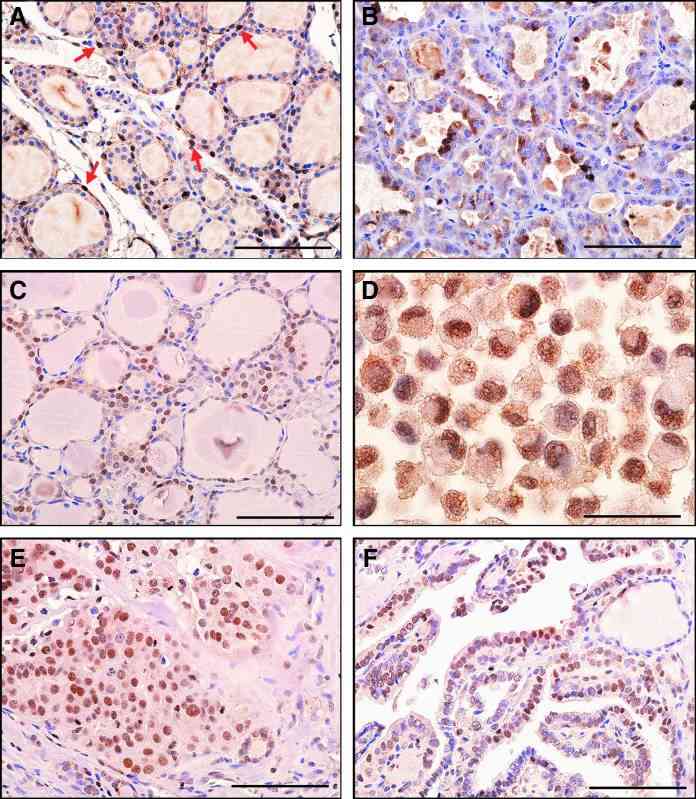 Fig. 4 STC1 antigen/protein expression in thyroid tissues. (A) Histology of normal mouse thyroid. (B) Mouse thyroid adenoma showing focal cytoplasmic staining. (C) Histology of normal human thyroid. Note nuclear staining. (D) Human RO82-W-1 thyroid carcinoma cell line (cell pellet) showing nuclear staining. (E) Human thyroid follicular carcinoma. (F) Human papillary thyroid carcinoma showing weak nuclear staining. (Hayase S, et al., 2015)
Fig. 4 STC1 antigen/protein expression in thyroid tissues. (A) Histology of normal mouse thyroid. (B) Mouse thyroid adenoma showing focal cytoplasmic staining. (C) Histology of normal human thyroid. Note nuclear staining. (D) Human RO82-W-1 thyroid carcinoma cell line (cell pellet) showing nuclear staining. (E) Human thyroid follicular carcinoma. (F) Human papillary thyroid carcinoma showing weak nuclear staining. (Hayase S, et al., 2015)
Description: Human papillary thyroid carcinoma cell line metastased to lymphnode. STR-PCR analysis...
Description: The TT cell line was established by S.S. Leong, et al. from a specimen obtained by...
Description: Derived from a primary papillary thyroid carcinoma. Retains thyroid follicular cell...

