Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
MEL-JUSO
Cat.No.: CSC-C0235
Species: Human
Source: melanoma
Morphology: epithelial-like adherent cells growing in monolayers
Culture Properties: monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Immunology: cytokeratin -, desmin -, endothel -, GFAP -, HMB-45 +, neurofilament -, vimentin +
Viruses: ELISA: reverse transcriptase negative; PCR: EBV -, HBV -, HCV -, H
Researchers established the MEL-JUSO cell line from a primary melanoma tumor taken from a 58-year-old woman in 1977. The cells demonstrate epithelial-like cell-cell adhesion while forming a single cell layer. Transplantation of MEL-JUSO cells into immunodeficient mice enables the creation of tumor models that researchers use to examine melanoma progression and dissemination. Proliferation and survival of this cell line depend on the ERBB4 and ERBB2 signaling pathways activation. Studies indicate that the P759L mutation in ERBB4 strongly promotes clonal expansion of MEL-JUSO cells while the K753A mutation in ERBB2 inhibits their cellular growth. The MEK/ERK signaling pathway activation in MEL-JUSO cells directly correlates with their response to drugs like 17-AAG and Cobimetinib.
Researchers frequently use the MEL-JUSO cell line to evaluate both the effectiveness and harmful effects of anti-cancer medications. Scientific research has revealed that Enoxacin and Ofloxacin produce substantial growth-inhibitory effects in MEL-JUSO cells. Furthermore, these cells serve as a research tool for studying how various drugs affect DNA methylation levels.
Extracellular Acidosis Leads to Reduced Proliferation and G1/G0 Cell Cycle Arrest
Solid tumors such as malignant melanoma produce acidosis within their microenvironment because of metabolic alterations that occur in cancer cells. Research demonstrates that acidosis produces varied outcomes which include promoting tumor invasiveness and metastasis as well as triggering cell apoptosis and autophagy. Böhme et al. focused on the effects of prolonged extracellular acidosis on melanoma cells, hypothesizing they are constantly subjected to an acidic environment in vivo. Three melanoma cell lines (Mel Im, SKMel28, and Mel Juso) were treated with a MES-acidified RPMI medium at pH 6.7 for over two months. Notably, prolonged acidosis led to morphological changes, like enlarged cell bodies and branched extensions in these cells (Fig. 1a, arrows). All tested cell lines showed a marked decrease in proliferation, indicating a slow-cycling phenotype (Fig. 1b, LT pH 6.7). No apoptosis was induced during this period. The use of flow cytometry combined with propidium iodide staining showed elevated numbers of cells in the G1/G0 phase with fewer S-phase cells which suggests that cells were arrested in the G1/G0 phase (Fig. 1c and d).
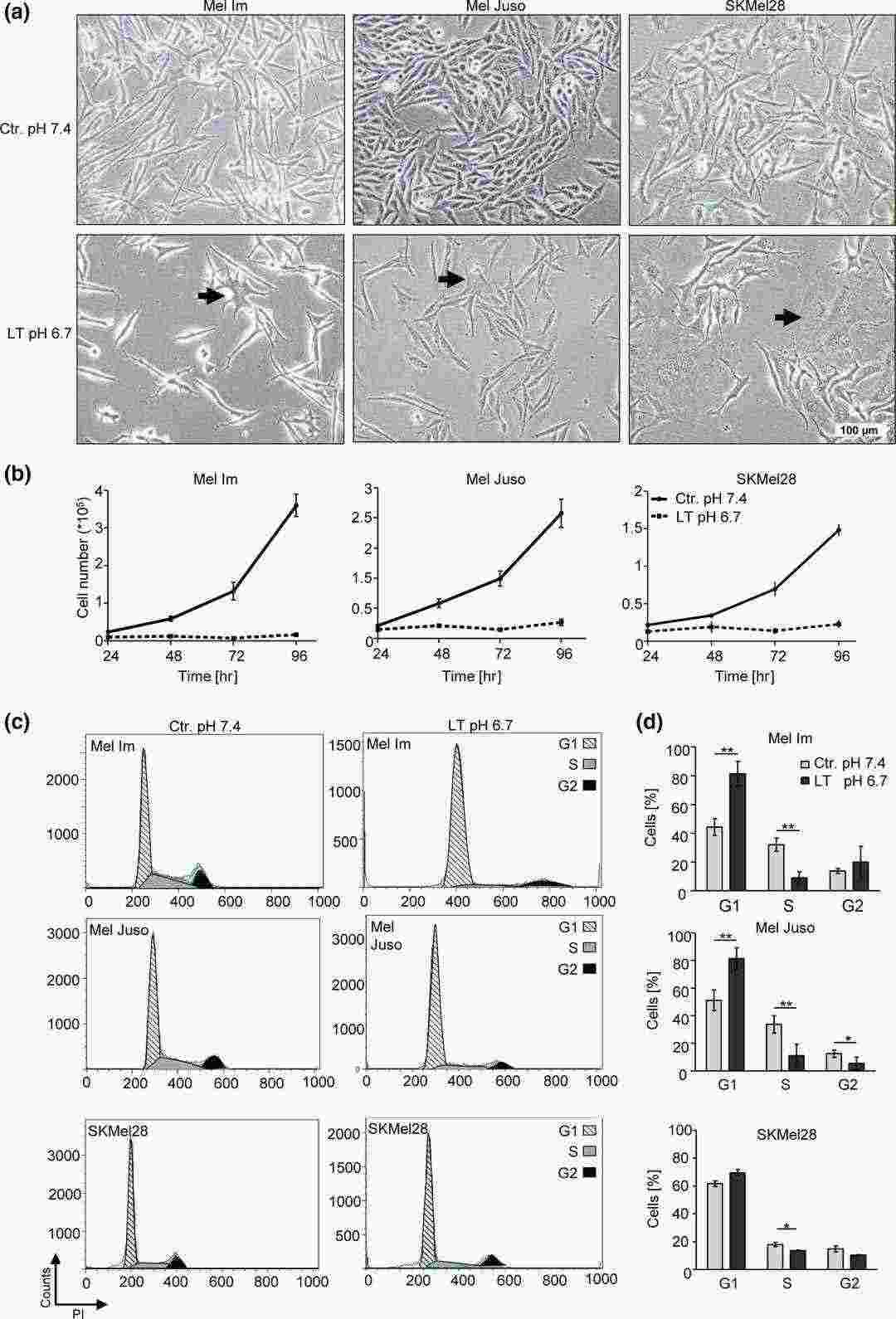 Fig. 1. Long-time acidosis mediates morphological alterations, reduction of proliferative activity, and G1/G0 cell cycle arrest (Böhme I and Bosserhoff A, 2019).
Fig. 1. Long-time acidosis mediates morphological alterations, reduction of proliferative activity, and G1/G0 cell cycle arrest (Böhme I and Bosserhoff A, 2019).
NO-SERM 4d Reduces Prometastatic Behavior of Melanoma Cells
The incidence of malignant melanoma is increasing, with survival rates declining significantly at advanced stages. Currently, available therapies often cause severe side effects and may lead to resistance. Estrogens potentially play roles in melanoma growth, influenced by ERα and ERβ pathways. Bechmann et al. used retrospective data analysis from TCGA to assess ER expressions in melanoma patients. It further evaluated the impact of ERβ-specific targeting through a nitric oxide-releasing selective ER modulator, NO-SERM 4d, on cellular behaviors linked to metastasis (Fig. 2a). Two melanoma cell lines, A2058 and MEL-JUSO, with similar ERα/ERβ levels but different receptor localizations, were used. A concentration of 10 μM NO-SERM 4d, known to not affect proliferation, was applied. Treatment significantly reduced motility, migration, and invasion in both cell lines (Fig. 2b–d). In A2058 cells, NO-SERM 4d significantly decreased adhesion to fibronectin, while MEL-JUSO showed a similar trend (Fig. 2e). These results suggest that inhibiting ERβ impacts various steps of the invasion–metastasis cascade in melanoma cells.
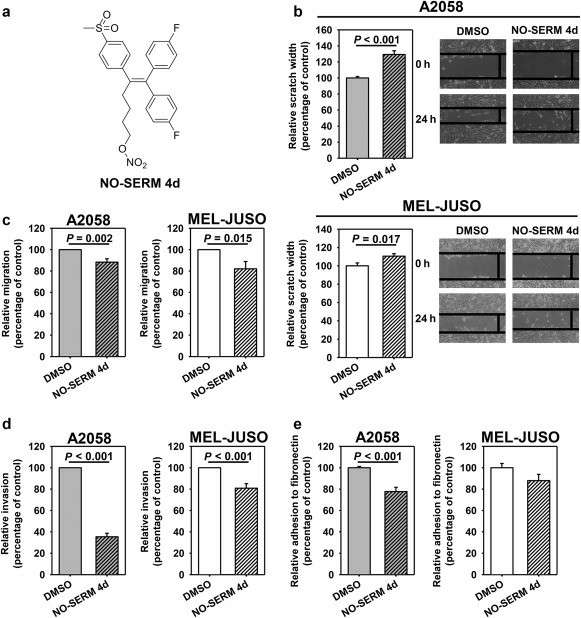 Fig. 2. Impact of NO-SERM 4d (10 μM) on prometastatic behavior of melanoma cell models (Bechmann N, Calsina B, et al., 2022).
Fig. 2. Impact of NO-SERM 4d (10 μM) on prometastatic behavior of melanoma cell models (Bechmann N, Calsina B, et al., 2022).
Ask a Question
Write your own review
- You May Also Need