Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Gastric Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
- FAQ
Background
Gastric tumor cells are the basis of gastric cancer (GC), which is one of the five most common malignancies and the third leading cause of cancer mortality worldwide. Despite the significant advancements in treatment, the prognosis for GC patients remains poor, primarily due to frequent relapse, metastasis, and drug resistance.
These cells are abnormal cells that grow uncontrollably in the stomach, forming a tumor. They can originate from different parts of the stomach, leading to different types of GC. The exact cause of their formation is not fully known, but it is believed to involve a combination of genetic factors, environmental factors, and infection with certain types of bacteria, such as Helicobacter pylori.
Gastric Tumor Cell Characteristics
- Cellular morphology and phenotypic features. Gastric tumor cells often exhibit altered cell shapes, sizes, and organization compared to normal gastric epithelial cells. They may display an increased nuclear-to-cytoplasmic ratio, irregular nuclear shapes, and prominent nucleoli. Gastric tumor cells can lose their polarized epithelial morphology and adopt a more dedifferentiated, mesenchymal-like phenotype. Aberrant expression of cell surface markers, such as adhesion molecules and growth factor receptors, is commonly observed in gastric tumor cells.
- Genetic and molecular alterations. Gastric tumor cells harbor various genetic and epigenetic changes that contribute to their malignant transformation. Commonly reported genetic alterations include mutations in tumor suppressor genes (e.g., TP53, ARID1A) and oncogenes (e.g., KRAS, HER2/ERBB2). Dysregulation of signaling pathways, such as the PI3K/AKT, RAS/MAPK, and Wnt/β-catenin pathways, is frequently observed in gastric tumor cells. Epigenetic modifications, including aberrant DNA methylation and histone modifications, can lead to the silencing of tumor suppressor genes and activation of oncogenes in gastric tumor cells.
- Tumor heterogeneity. Gastric tumors exhibit substantial heterogeneity both between different GC subtypes and within individual tumors. This heterogeneity is reflected in the diverse morphological, genetic, and phenotypic characteristics of gastric tumor cells.
In Vitro Cell Line Models
One of the primary applications of gastric tumor cells lies in the development and utilization of in vitro cell line models. These models, established from the tumor cells of GC patients, serve as invaluable tools for researchers.
By cultivating and maintaining these cell lines, scientists can closely study the behavior and properties of gastric tumor cells under controlled laboratory conditions. This allows for a deeper exploration of the underlying mechanisms that drive their proliferation, invasion, and metastasis, as well as the evaluation of the efficacy and specificity of potential anti-cancer compounds. The availability of these well-characterized gastric tumor cell lines has been instrumental in accelerating the drug discovery and development process, as they provide a reliable platform for high-throughput screening and preclinical testing of novel therapeutic interventions.
Biomarker Identification
Gastric tumor cells also play a crucial role in the identification and validation of biomarkers for GC. By analyzing the genetic, molecular, and phenotypic profiles of these cells, researchers have been able to uncover a range of potential diagnostic and prognostic markers. For instance, detecting specific genetic alterations, such as mutations in TP53 or HER2 amplification, in gastric tumor cells can serve as valuable indicators for disease diagnosis and risk stratification. Furthermore, the expression patterns of cell surface markers and secreted factors by these cells can provide insights into the progression and metastatic potential of gastric tumors.
Advancing Cancer Research
Beyond the clinical applications, gastric tumor cells have also been instrumental in advancing the fundamental understanding of cancer biology. Investigations into the signaling pathways, epigenetic modifications, and the tumor microenvironment factors that influence gastric tumor cell behavior have led to groundbreaking discoveries. These insights have not only expanded our understanding of GC but have also informed research on other types of malignancies, fostering a more comprehensive approach to cancer research and treatment development.
Cell State-Specific Transcriptional Patterns Associated With GC Uncovered by scRNA-seq
To examine tumor-associated expression programs at the single-cell level, primary gastric tumor samples were compared to normal tissues (n = 26 tumor; n = 10 normal). After random downsampling to ensure statistical equivalency (Fig. 1A), the proportion of epithelial cells in tumors was lower compared with normal tissues (P = 0.002), whereas myeloid cells in tumors were higher (P = 0.05). The proportions of lymphoid, plasma, and stromal cells were not statistically different between tumor and normal samples.
Compared with their cognate cell states in normal tissues, tumor-associated epithelial cells exhibited higher expression of multiple oncogenic gene signatures related to epithelial-mesenchymal transition (EMT), cell motility, and cancer signatures derived from previous GC single-cell RNA sequencing (scRNA-seq) studies (Fig. 1B; P < 0.0001). When the expression status of individual GC oncogenes was assessed (e.g., CEACAM6, EGFR, MET, CCND1, and KRAS) among the tumor epithelial clusters, the EpiInt1 tumor epithelial cluster exhibited the largest increase (Fig. 1C). Comparisons in a cell state-specific manner revealed that "chief cells" and "intestinal-type cells" showed the greatest differences in transcriptomic profiles between GC and their cognate cell state in normal gastric tissues (Fig. 1E; "chief cells": 1,232 genes/EpiC3 cluster; "intestinal-type cells": 775 genes/EpiInt2 cluster). Pathway analysis indicated that each tumor epithelial cluster shared both common upregulated modules relevant to cancer, but also cluster-specific tumor-associated modules. Among downregulated genes, the classic chief cell markers LIPF and PGA3 were among the top downregulated genes in tumor epithelial cells (Fig. 1F; P < 0.0001). Using spatial DSP, the loss of LIPF was confirmed in tumor epithelial cells (Pan-CK+) compared with normal samples (n = 13, P = 0.0035; Fig. 1G).
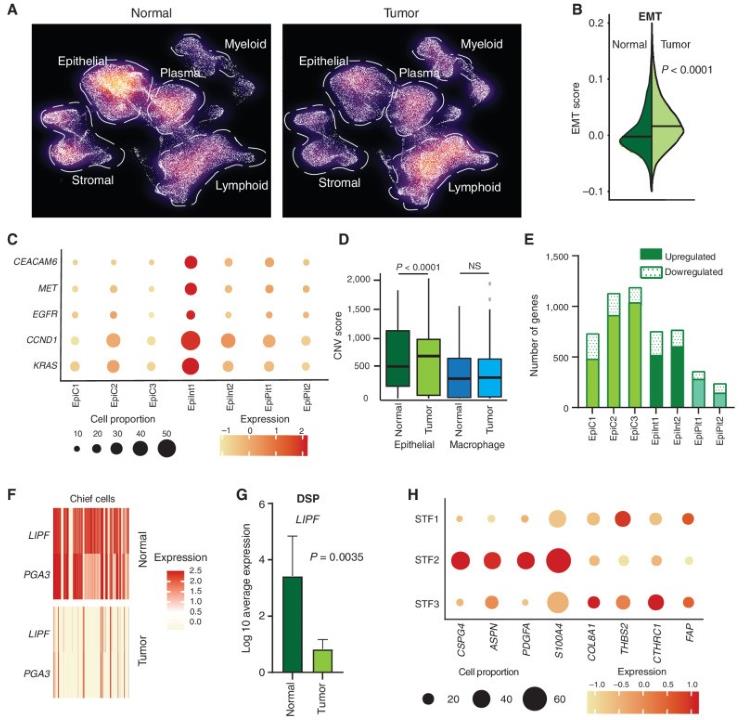 Fig. 1 scRNA-seq deconvolutes gastric tumor programs associated with distinct cell states. (Kumar V, et al, 2022)
Fig. 1 scRNA-seq deconvolutes gastric tumor programs associated with distinct cell states. (Kumar V, et al, 2022)
Neutrophils Promote Migration and Invasiveness and EMT of GC Cells
To study the effect of neutrophils on GC cells, a Transwell migration/invasion assay was used to explore the effect of neutrophils on the migration and invasion ability of GC cells. The results showed that neutrophils promote GC cell (MKN45 and MKN74) migration (P < 0.001 and P < 0.001) and invasion (P < 0.001 and P < 0.001) (Fig. 2a and b), when an IL-17a neutralizing antibody was added to the Transwell co-culture chamber, GC cell (MKN45 and MKN74) migration (P < 0.001 and P < 0.001) and invasion was decreased (P < 0.001 and P < 0.001) (Fig. 2a and b). Therefore, neutrophil promotes the migration and invasiveness of GC cells through IL-17a.
Western blot results showed that neutrophils promote the expression of EMT-related markers in GC cells (MKN45 and MKN74). E-cadherin expression was significantly decreased (P < 0.05 and P < 0.05 in MKN45 and MKN74 cells, respectively), and Vimentin (P < 0.05 and P < 0.05 in MKN45 and MKN74 cells, respectively) and ZEB1 (P < 0.05 and P < 0.05 in MKN45 and MKN74 cells, respectively) expression was significantly upregulated (Fig. 2c) when an IL-17a neutralizing antibody was added to the Transwell co-culture system, The expression of E-cadherin protein in the epithelium of GC cells was upregulated (P < 0.05 and P < 0.05 in MKN45 and MKN74 cells, respectively), while the expression of Vimentin protein in the interstitial space decreased significantly (P < 0.05 and P < 0.05 in MKN45 and MKN74 cells, respectively). The expression of the transcription factor ZEB1 was also significantly downregulated (P < 0.05 and P < 0.05 in MKN45 and MKN74 cells, respectively) (Fig. 2c). Therefore, neutrophils promote EMT in GC cells, and further promote the migration and invasiveness of GC cells, which may be mediated by IL-17a.
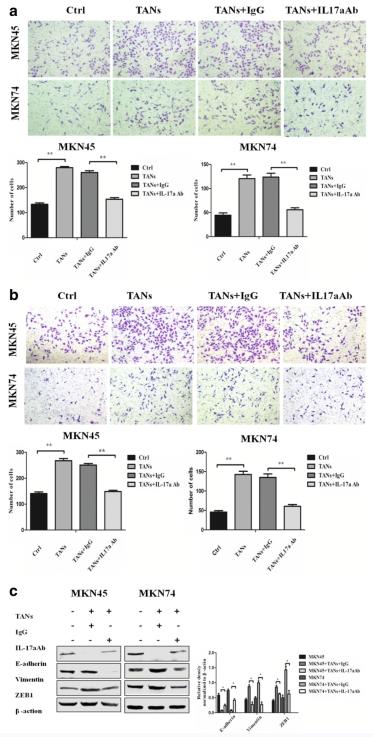 Fig. 2 Neutrophils enhance the migration, invasiveness, and EMT of GC cells through IL-17a. (Li S, et al., 2019)
Fig. 2 Neutrophils enhance the migration, invasiveness, and EMT of GC cells through IL-17a. (Li S, et al., 2019)
Gastric tumor cell lines are established by isolating and culturing cells directly from the tumors of GC patients. These cell lines are extensively characterized to ensure they faithfully represent the properties of the original tumors.
These include uncontrolled proliferation and disregard for normal growth signals; increased invasive and metastatic behavior; altered gene expression profiles, such as mutations in key oncogenes and tumor suppressor genes; metabolic reprogramming to support rapid growth and survival; increased expression of cell surface markers associated with cancer stem cells.
Some of the critical signaling pathways involved in this process include PI3K/Akt/mTOR, Ras/MAPK, Wnt/β-catenin, Notch signaling, and TGF-β/Smad pathways.
Description: Small-cell gastrointestinal carcinoma. High levels of creatine kinase brain isoenzyme. Cell growth is slow.
Description: Small cell gastrointestinal carcinoma with c-myc overexpression. Cell growth is slow.
Description: Stomach cancer (Borrmann IV). Mucin, CA19-9, CEA, alphafetoprotein positive Cell growth is slow.
Description: established from brachialis muscle metastasis of a 72-year-old male patient with gastric adenocarcinoma
Description: established from omentum metastasis of a 46-year-old man with gastric choriocarcinoma
Description: Species: human - male, 72 years old, Mongoloid
Description: Species: human - female, 70 years old, Mongoloid
Histopathology: adenocarcinoma, tubular
Description: Species: human - male, 37 years old, Mongoloid
Histopathology: adenocarcinoma, tubular

