Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Immortalized Human Skeletal Muscle Myoblasts
Cat.No.: CSC-I9102L
Species: homo sapiens
Source: Skeletal Muscle
Morphology: Multipolar
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
Skeletal muscle is the largest tissue, accounting for approximately 40% of the human body mass. Not only does it provide the mechanism behind movement, but it also plays a crucial role in metabolism and regulating immune system functions. Skeletal muscle is susceptible to a plethora of genetic and metabolic disorders, while also undergoing loss in cancer and aging, making it one of the main targets for regenerative medicine. Therefore, it is becoming essential to establish a well-characterized and reproducible in vitro model of human skeletal muscle to gain insight into the development, diseases and regeneration of this tissue and to help identify novel therapeutic interventions.
One of the most widely used in vitro systems for studying muscle pathophysiology is mouse myoblast culture, which differentiates into mature myotubes with spontaneous contractile capacity within 6-14 days. In contrast, human skeletal myotubes exhibit lower maturation and survival capacity with reduced sarcomeric cross-striation, and they generally lack contractile capacity. Moreover, sample availability is a substantial limiting factor due to scarcity of muscle biopsies and limited proliferation of human myoblasts.
On the other hand, in vivo mouse models often fail to reproduce the severity of human disorders, which may be due to the reduced regeneration capacity of human muscles compared to mice. Furthermore, different underlying pathophysiological mechanisms in humans and mice cannot be excluded, and thus, therapies tested on mouse models may not necessarily yield the same results in human patients.
More recently, significant progress has been made toward engineering functional 3D human muscle systems using iPSCs. While these models overcome limitations in sample availability and have demonstrated physiological relevance to humans, still they require special handling and are resource-intensive in terms of cost and time. Immortalization of primary myoblasts seems to be a reasonable compromise among sample availability, relevance to human muscle, and cost-effectiveness.
Immortalized Myogenic Cell Lines Generated from Duchenne Muscular Dystrophy, Congenital Muscular Dystrophies and Control Muscles
In Duchenne muscular dystrophy (DMD) patients, absence of dystrophin causes muscle wasting by impacting myofiber integrity and the properties of muscle stem cells (MuSCs). However, DMD-derived MuSC usage is restricted by the limited number of divisions that human MuSCs can undertake in vitro before losing their myogenic characteristics and by the scarcity of human material available from DMD muscle. To overcome these limitations, immortalization of MuSCs (iHMuSC) appears as a strategy.
Here, we derived MuSC clones from a series of clinically and genetically characterized patients, including eight DMD patients with various mutations, four congenital muscular dystrophies (CMD) and three age-matched control muscles. The myogenic characteristics and differentiation potential were tested for each clone. Figure 1 (2D-glass) shows examples of iHMuSCs forming myotubes on a 2D glass culture support. Figure 1 (3D-matrigel) shows that iHMuSCs from either control, CMD or DMD patients formed large elongated myotubes containing numerous nuclei and having developed their contractile apparatus, as assessed by MHC expression.
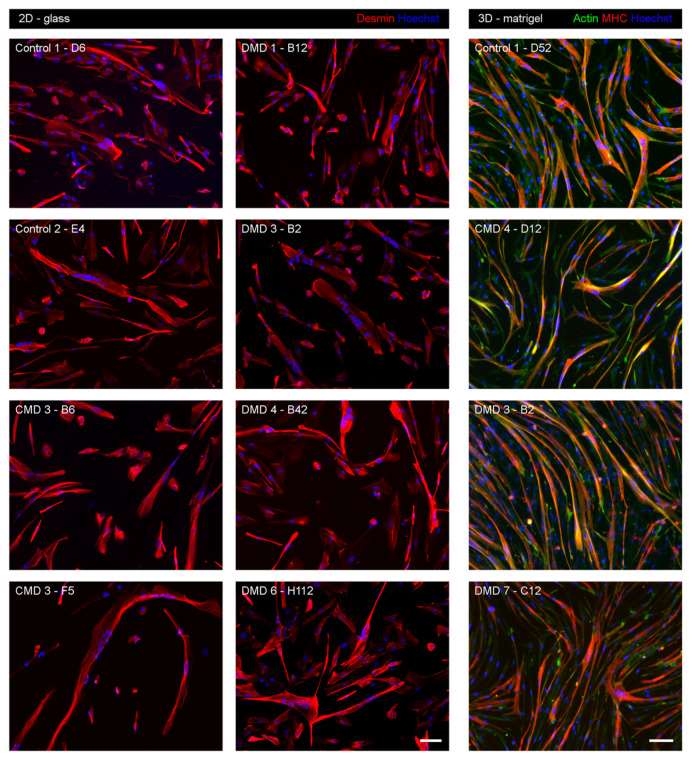 Fig. 1. Myogenic differentiation of iHMuSC clones (Massenet, Jimmy, et al. 2020).
Fig. 1. Myogenic differentiation of iHMuSC clones (Massenet, Jimmy, et al. 2020).
Primary Human Myoblast Immortalization After Lentiviral CDK4 and hTERT Transduction
Primary myoblast cells were transduced with lentiviral vectors encoding the cyclin-dependent kinase 4 (CDK4) and human telomerase reverse transcriptase (hTERT) proteins, two factors that were shown to be sufficient to immortalize human myoblast cells while maintaining a differentiation potential and stable karyotype. While primary myoblast cells stopped proliferating after 45 days, cells transduced with CDK4 and hTERT continued growing stably for up to 6 months (Fig. 2A). Immortalized myoblasts were differentiated in vitro and analyzed by immunofluorescence staining of myocyte enhancer factor-2 (MEF2) and myosin heavy chain (MyHC) proteins to determine their ability to fuse into multi-nucleated myotubes. We observed that most cells fused into myotubes (MyHC-positive cells) expressing MEF2, confirming that transduction of primary myoblasts with lentiviral vectors encoding the CDK4 and hTERT proteins can efficiently immortalize cells without affecting their myogenicity (Fig. 2B).
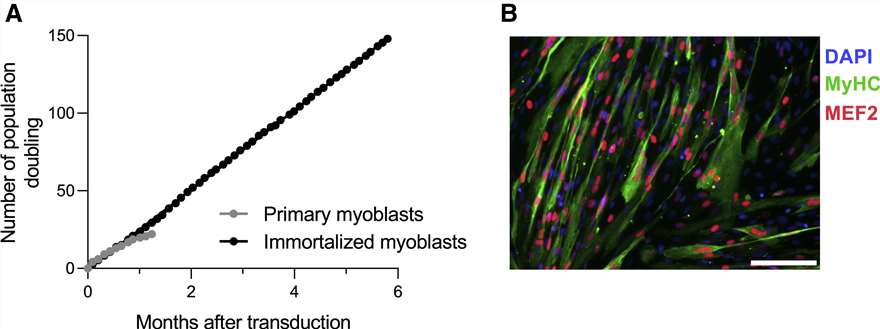 Fig. 2. Primary myoblast cells were transduced with lentiviral vectors encoding the CDK4 and hTERT proteins (Lathuiliere, Aurelien, et al. 2022).
Fig. 2. Primary myoblast cells were transduced with lentiviral vectors encoding the CDK4 and hTERT proteins (Lathuiliere, Aurelien, et al. 2022).
Each order includes a Certificate of Analysis, detailed usage instructions, and a culture manual. Additionally, our experienced technical support team is available to assist with any questions or challenges that may arise during your research.
Yes, Immortalized Human Skeletal Muscle Myoblasts-SV40T are capable of differentiating into myotubes. This process requires the use of a specialized differentiation medium, which is available for purchase separately.
Ask a Question
Write your own review