Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Immortalized Human Fallopian Tube Secretory Epithelial Cells (FT33-shp53-R24C)
Cat.No.: CSC-I9204L
Species: homo sapiens
Source: Fimbriae of fallopian tube
Morphology: Cobblestone-like
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
The FT33-shp53-R24C cell line originates from secretory epithelial cells located in the human fallopian tube fimbriae which serve as essential elements of the tube's inner lining composed primarily of secretory and ciliated cells. Fallopian tube secretory epithelial cells are essential to reproductive processes because they release fluids that help move sperm and ova while supporting fertilization nutritionally. Additionally, studies suggest that these cells serve as potential origin points for high-grade serous ovarian carcinoma (HGSOC).
FT33-shp53-R24C cells exhibit the typical cuboidal or low columnar morphology, similar to their in vivo secretory epithelial cell counterparts. These cells demonstrate good adhesion and proliferative abilities under culture conditions, making them suitable for long-term cultivation. FT33-shp53-R24C cells retain the expression of Müllerian markers of fallopian tube secretory epithelial cells, such as PAX8 and CK7. Moreover, through shRNA technology, the TP53 gene has been knocked down, and the CDK4 gene with a p.Arg24Cys mutation, along with the TERT gene, has been integrated. This genetic modification allows the cells to maintain secretory epithelial cell characteristics while circumventing p53-related senescence and apoptosis mechanisms. As fallopian tube secretory epithelial cells, the FT33-shp53-R24C cell line retains the function of secreting substances like mucus, aiding in the simulation of the fallopian tube's physiological environment. Researchers can study both normal and abnormal functions of fallopian tube epithelial cells with this cell line which is crucial for understanding ovarian cancer development mechanisms.
CLF Synergizes with Olaparib in FT33 Cells Transformed with TAg-Myc or shP53/CDK4R24C
Drug-resistant ovarian cancer presents a significant treatment challenge. Enhancing sensitivity to PARP inhibitors could improve therapeutic outcomes. Thirsangu et al. explored using clofarabine (CLF) to sensitize ovarian cancer cells to PARP inhibitors like olaparib, utilizing cell lines, patient-derived cultures, primary tumors, and xenografts.
In their efforts to enhance sensitivity to PARP inhibitors, they identified clofarabine (CLF) as a potential therapy for drug-resistant ovarian cancer and nuclear trafficking of Cathepsin L (CTSL) as a treatment- responsive biomarker. Then, to assess the toxicity of the CLF+olaparib combination in normal cells, they conducted a clonogenic assay with FT33 cells derived from non-diseased Fallopian tube secretory epithelial cells, immortalized by hTERT and SV40 antigens. Cells with c-Myc or sh-p53 and mutant CDK4 (CDK4R24C) transformations were tested. FT33, FT33-shP53/CDK4R24C, and FT33 c-Myc/TAg cells resisted CLF monotherapy up to 20nM (Fig. 1A). In FT33 cells, increased olaparib+5 nM CLF showed resistance (Fig. 1B), but transformed FT33-shP53-R24C and FT33-TAg-Myc increased sensitivity to olaparib (Fig. 1C and D). CLF+olaparib boosted nCTSL translocation only in transformed cells, indicating drug-induced nCTSL as a biomarker for response in transformed tumor cells. This combination enhances antitumor effects and DNA damage response modulation in preclinical OC models.
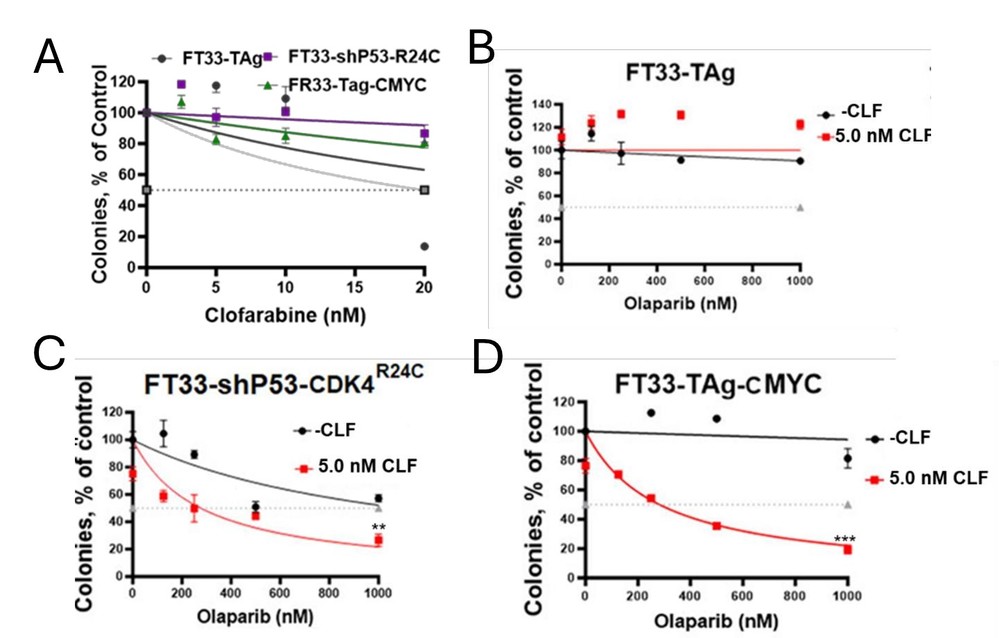 Fig. 1. Cytotoxicity assessment by colony forming assay upon treatment with clofarabine alone in FT33-TAg, FT33-shP53-CDK4(R24C) and FT33-TAg-cMYC in (A) and with – and + 5 nM CLF in combination with olaparib at indicated concentration in (B) FT33-TAg, (C) FT33-shP53-CDK4(R24C) and (D) FT33-TAg-cMYC (Thirsangu P, Jin L, et al., 2025).
Fig. 1. Cytotoxicity assessment by colony forming assay upon treatment with clofarabine alone in FT33-TAg, FT33-shP53-CDK4(R24C) and FT33-TAg-cMYC in (A) and with – and + 5 nM CLF in combination with olaparib at indicated concentration in (B) FT33-TAg, (C) FT33-shP53-CDK4(R24C) and (D) FT33-TAg-cMYC (Thirsangu P, Jin L, et al., 2025).
XPO1's Nuclear Localization in Ovarian Cancer Cell Lines
The treatment resistance of ovarian cancer to platinum chemotherapy leads to high mortality rates among patients. The proper functioning of nuclear-cytoplasmic transport becomes disrupted in cancer cells while exportin-1 (XPO1) plays a vital role in this process. Many cancers show XPO1 overexpression which affects cancer advancement proteins while also promoting chemoresistance especially in ovarian cancer cases.
Chen et al. quantified XPO1 protein levels and subcellular localization. First, they examined XPO1 expression in four well-characterized and frequently studied immortalized ovarian cancer cell lines (A2780, CP70, OVCAR3, and SKOV3) and compared these expression levels with other ovarian- and non-ovarian–derived cell lines. Western blot analysis demonstrated consistently higher levels of XPO1 expression in the four ovarian cancer cell lines compared with noncancerous ovarian surface epithelial cells (IOSE527), a fallopian tube cell line (FT33-shp53-R24C), and four fibroblast cell lines (IMR-90, Wi-38, AG060062, and GM17071A; Fig. 2B).
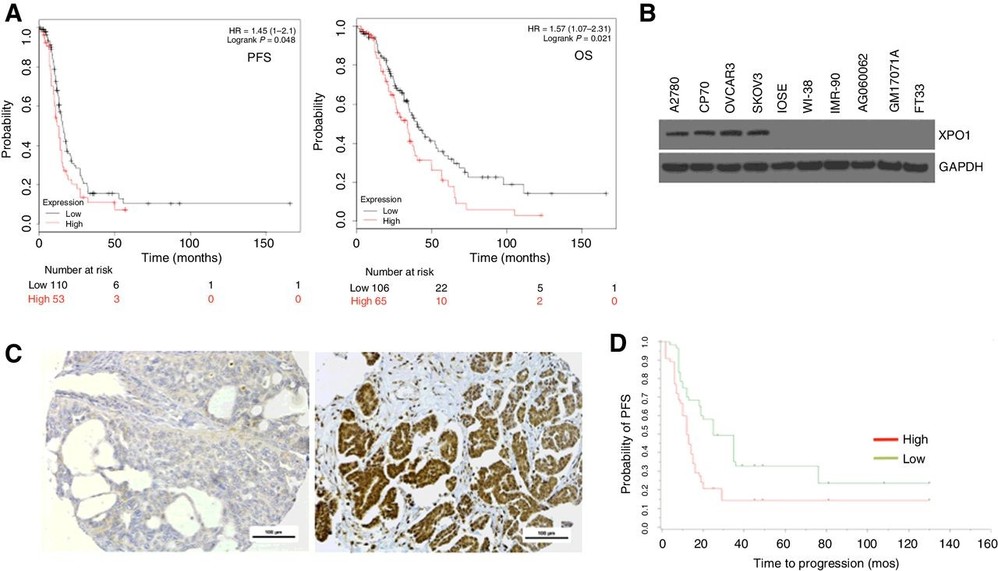 Fig. 2. XPO1 is highly expressed in human ovarian cancer, and expression is associated with worse survival (Chen Y; Camacho S C, et al., 2017).
Fig. 2. XPO1 is highly expressed in human ovarian cancer, and expression is associated with worse survival (Chen Y; Camacho S C, et al., 2017).
Yes, Immortalized Human Fallopian Tube Secretory Epithelial Cells can be cryopreserved for long-term storage. Following established freezing protocols helps maintain cell viability upon thawing. It's essential to carefully follow guidelines for freezing and thawing to preserve cell health.
Yes, these cells can be utilized for drug testing and toxicity studies related to reproductive health. Their immortalized nature and similarity to human tissue make them an excellent choice for screening potential therapeutic compounds.
Ask a Question
Write your own review

