Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Immortalized Human Coronary Artery Endothelial Cells
Cat.No.: CSC-I9142L
Species: Homo sapiens
Source: Heart
Morphology: Polygonal
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
Immortalized human coronary artery endothelial cells is a cell line generated from human coronary artery endothelial cells via transfection of SV40 DNA. These cells not only maintain the core features of the primordial endothelial cells, including endothelial markers such as Ulex europaeus I lectin and factor VIII-associated antigen, but they can also generate some very important bioactive molecules, such as prostacyclin, tissue-type plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1).
Because they are capable of modelling angiogenesis in vitro, immortalized cells hold tremendous promise for drug testing and cardiovascular disease research. They can be used to better understand the underlying causes of cardiovascular diseases, including coronary atherosclerosis, myocardial infarction and coronary heart disease. These include exploring the primary pathways of disease onset and progression in endothelial dysfunction, inflammation, oxidative stress and disorders of lipid metabolism. Additionally, these cells help to discover early diagnostic markers and therapeutic targets for disease. To better mimic the pathology of atherosclerosis in vivo, scientists can generate an in vitro atherosclerosis model by injecting molecules such as oxidized low-density lipoprotein and inflammatory factors into these immortalized cells. This model not only improves the way we understand how atherosclerosis progresses but also offers an invaluable experimental platform for bringing novel medications to market.
Single Irradiation Induces Changes in the Gene Expression of Atheroprotective Cx37 and Cx40 and Proatherogenic Cx43
Radiotherapy has been linked to increased cardiovascular disease (CVD) risk, notably atherosclerosis, due to endothelial dysfunction post-ionizing radiation. Connexin (Cx) proteins in endothelial cells, forming gap junctions and hemichannels, play a crucial role in mediating these effects. Ramadan's team exposed immortalized human coronary artery endothelial cells (TICAE) and immortalized human dermal microvascular endothelial cells (TIME) to X-rays to analyze Cx expression and channel functions.
TICAE and TIME cells were exposed to X-ray doses (0.1, 0.5, and 5 Gy) to study changes in Cx gene expression over time (6h to 14d). In TICAE cells, Cx37 expression decreased from 24h to 14d, significant at 0.5 and 5 Gy. At 0.1 Gy, it increased at 6h but decreased by 7d (Fig. 1a). For TIME cells, 5 Gy reduced Cx37 expression at 24h and 14d but increased it at 48h. Doses of 0.1 and 0.5 Gy increased expression at 6h and 48h, respectively (Fig. 1a). Cx40 expression in TICAE cells decreased from 6h to 7d at 5 Gy, while 0.1 Gy increased it at 6h and 48h (Fig. 1b). In TIME cells, Cx40 increased early at 6h for 0.1 Gy and at 48h for higher doses, with a dose-dependent decrease from 72h to 14d at 5 Gy (Fig. 1b). Cx43 expression largely increased at 0.5 and 5 Gy. In TICAE, 5 Gy caused increases from 6h to 7d, while at 0.5 Gy, it normalized faster (Fig. 1c). In TIME cells, 5 Gy significantly increased Cx43 at 24h, 72h, and 14d, whereas lower doses showed no major changes.
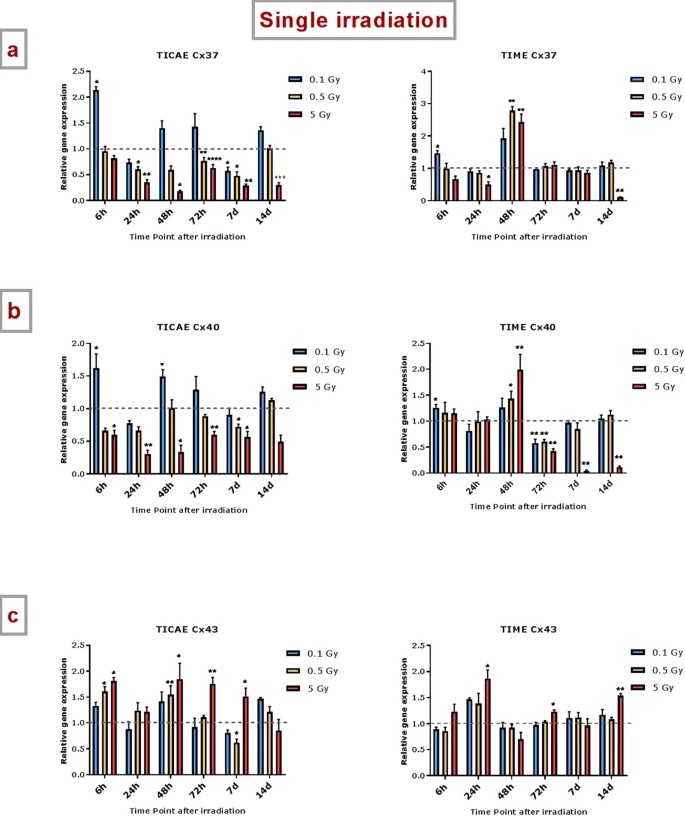 Fig. 1. The effect of single and fractionated irradiation on Cx37, Cx40 and Cx43 gene expression (Ramadan R, Vromans E, et al., 2019).
Fig. 1. The effect of single and fractionated irradiation on Cx37, Cx40 and Cx43 gene expression (Ramadan R, Vromans E, et al., 2019).
TAT-Gap19 Protects Against Radiation-Induced Cell Death
Radiotherapy in thoracic cancer patients elevates cardiovascular disease risk, notably atherosclerosis, through IR-induced endothelial dysfunction. Ramadan's team examined the effects of X-ray irradiation on human coronary/microvascular endothelial cells, focusing on Cx43 hemichannels.
To study IR-induced cell death, Annexin V and Caspase 3/7 activities were monitored in immortalized human coronary artery endothelial cells (TICAE) and immortalized human dermal microvascular endothelial cells (TIME) up to 100h post-IR using Incucyte imaging, with hemichannel inhibition assessed using TAT-Gap19. In TICAE cells, a significant increase in Caspase 3/7 activity and Annexin V was observed at 5 Gy dose from 4h to 100h post-IR (Fig. 2A and B). TAT-Gap19 reduced these activities at 5 Gy dose from 12h onward, and at lower doses from 16h onward for Caspase 3/7 and starting from 4-8h for Annexin V (Fig. 2A and B). In TIME cells, no significant Caspase 3/7 changes were noted at 0.1 and 5 Gy (Fig. 2D), but Annexin V increased significantly at 5 Gy from 4h onward (Fig. 2C), reduced by TAT-Gap19 during 4-48h. TAT-Gap19 also reduced Annexin V transiently at 0 Gy (12-24h; Fig. 2C). To further validate cell death in TIME cells, dextran fluorescein staining was assessed at 6 and 72h post-IR. Increased staining was observed at 5 Gy at 6h and at both 0.1 and 5 Gy at 72h (Fig. 2E and F), inhibited by TAT-Gap19. A minor increase with TAT-Gap19 was noted at 6h for control and 0.1 Gy (Fig. 2E). They also evaluated the effect of TAT peptide alone (100 μm) on cell death in TICAE and TIME cells. Results showed no significant effects on TICAE cells and only slightly increased Annexin V in TIME cells between 24-30h (Fig. 3).
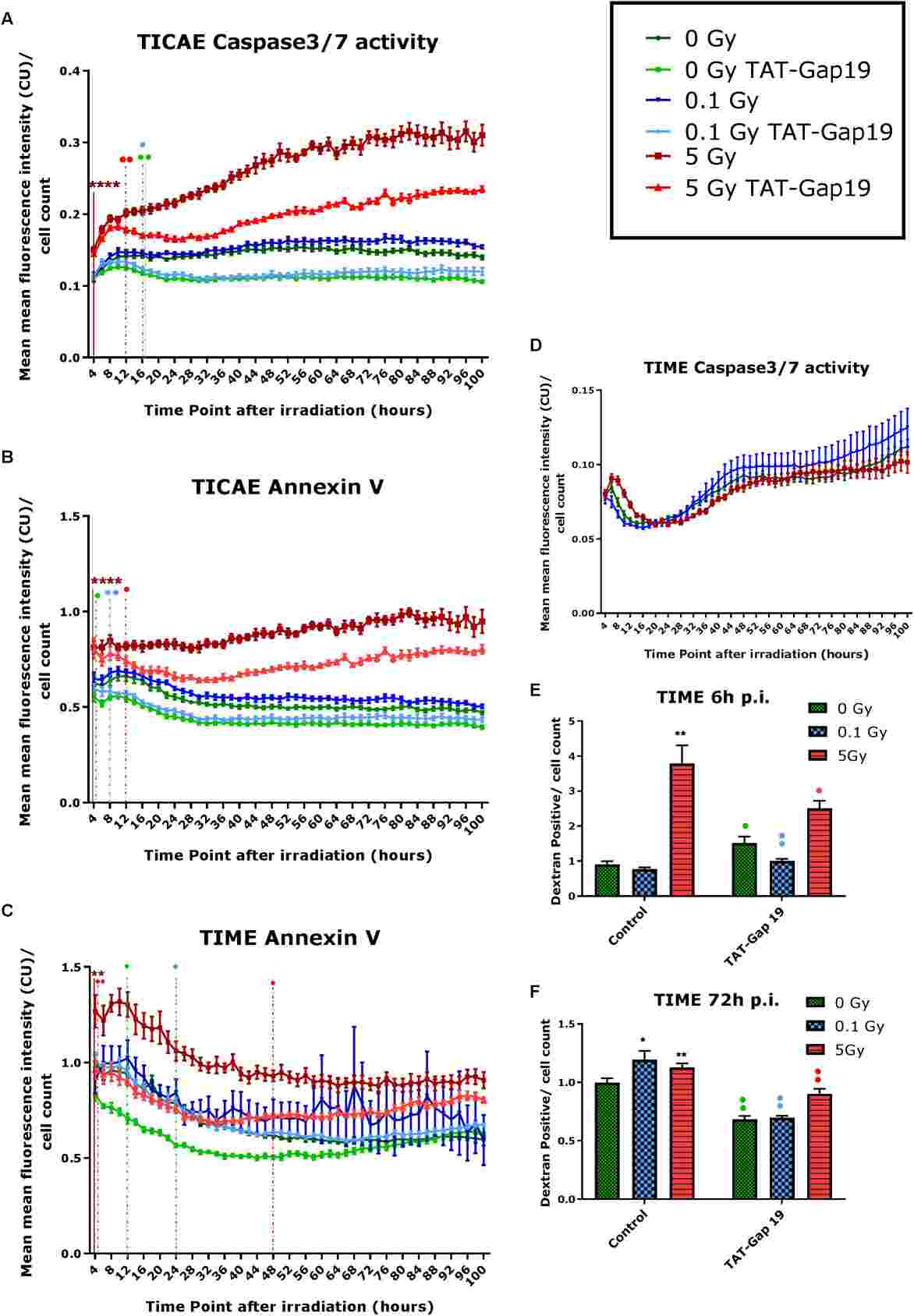 Fig. 2. Radiation-induced cell death as assessed by Caspase 3/7 activity, Annexin V and 10 kDa dextran fluorescein staining, and effect of TAT-Gap19 (Ramadan R, Vromans E, et al., 2020).
Fig. 2. Radiation-induced cell death as assessed by Caspase 3/7 activity, Annexin V and 10 kDa dextran fluorescein staining, and effect of TAT-Gap19 (Ramadan R, Vromans E, et al., 2020).
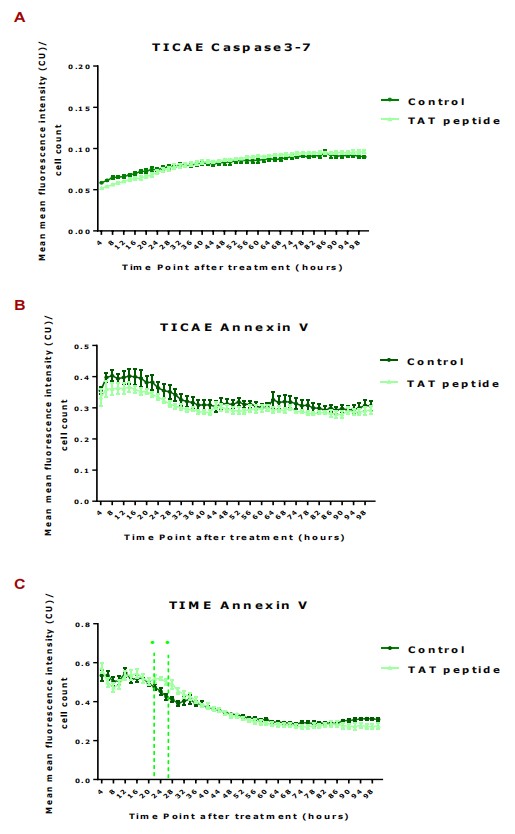 Fig. 3. Caspase 3/7 activity was assessed in TICAE cell and Annexin V was assessed in TICAE and TIME cells from 4 h until 100 h of 100 µl of TAT peptide exposure (Ramadan R, Vromans E, et al., 2020).
Fig. 3. Caspase 3/7 activity was assessed in TICAE cell and Annexin V was assessed in TICAE and TIME cells from 4 h until 100 h of 100 µl of TAT peptide exposure (Ramadan R, Vromans E, et al., 2020).
Ask a Question
Write your own review

