Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY

Immortalized Human Bronchial Epithelial Cells (HBE1)
Cat.No.: CSC-I9341L
Species: Homo sapiens
Source: Bronchi
Morphology: Epithelial-like
Culture Properties: Adherent
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Note: Never can cells be kept at -20 °C.
CIK-HT013 HT® Lenti-hTERT Immortalization Kit
CIK-HT003 HT® Lenti-SV40T Immortalization Kit
Immortalized Human Bronchial Epithelial Cells (HBE1) is derived from a 60-year-old female donor suffering from idiopathic pulmonary fibrosis and immortalized by transfection with the E6 and E7 genes of HPV in primary culture. The HBE1 cell line protects the main functional properties of human bronchial epithelial cells which involve mucus secretion, barrier maintenance and immune response participation. Studies indicate that HBE1 cells display markers which characterize multiple lung epithelial cell types including central and distal airway epithelial cells. Additionally, these cells have the ability to detect external stimuli like IL-17A while controlling gene expression.
HBE1 cells demonstrate the ability to replicate multiple pulmonary disease conditions. Consequently, they are widely used in research on airway diseases such as chronic obstructive pulmonary disease (COPD), asthma, and non-small cell lung cancer (NSCLC). Through the simulation of inflammatory responses or exposure to infections and pharmaceutical treatments researchers can understand disease mechanisms and test therapeutic approaches. Pulmonary regenerative medicine research benefits from the multipotent characteristics of HBE1 cells. Research shows these cells can develop into multiple lung cell types using three-dimensional culture systems which creates promising opportunities for lung tissue engineering.
PARP1 Inhibitor Suppressed NM-Induced HBE Cell Death
Sulfur mustard and nitrogen mustard are hazardous agents causing lung damage by inducing cell death. The JNK/c-Jun pathway is suspected of mediating this process, with questions on its specific role. Using immortalized human bronchial epithelial (HBE) cells exposed to nitrogen mustard, Ye's team established an in vitro model to examine JNK/c-Jun activation.
They tested HBE cells with three different NM concentrations of 3 μM, 7 μM, and 20 μM to evaluate their toxic effects. Cell viability experienced a reduction to 91% after 10 hours and to 66% after 24 hours when exposed to 20 μM NM (Fig. 1A). The Western blot analysis revealed that caspase-3, caspase-7, phosphorylated H2AX at S139 site, PARP1 along with c-Jun and phosphorylated c-Jun all reached their highest levels at the 24-hour mark following treatment (Fig. 1B). The markers p-H2AX and c-Jun, as well as phosphorylated c-Jun showed earlier responses at reduced doses. The PARP1 inhibitor veliparib at a concentration of 50 μM blocked viability reduction while ATP levels remained stable (Fig. 1C and D). YO-PRO-1 and PI staining indicated increased apoptosis and necrosis in NM-injured cells, reduced by veliparib (Fig. 1E).
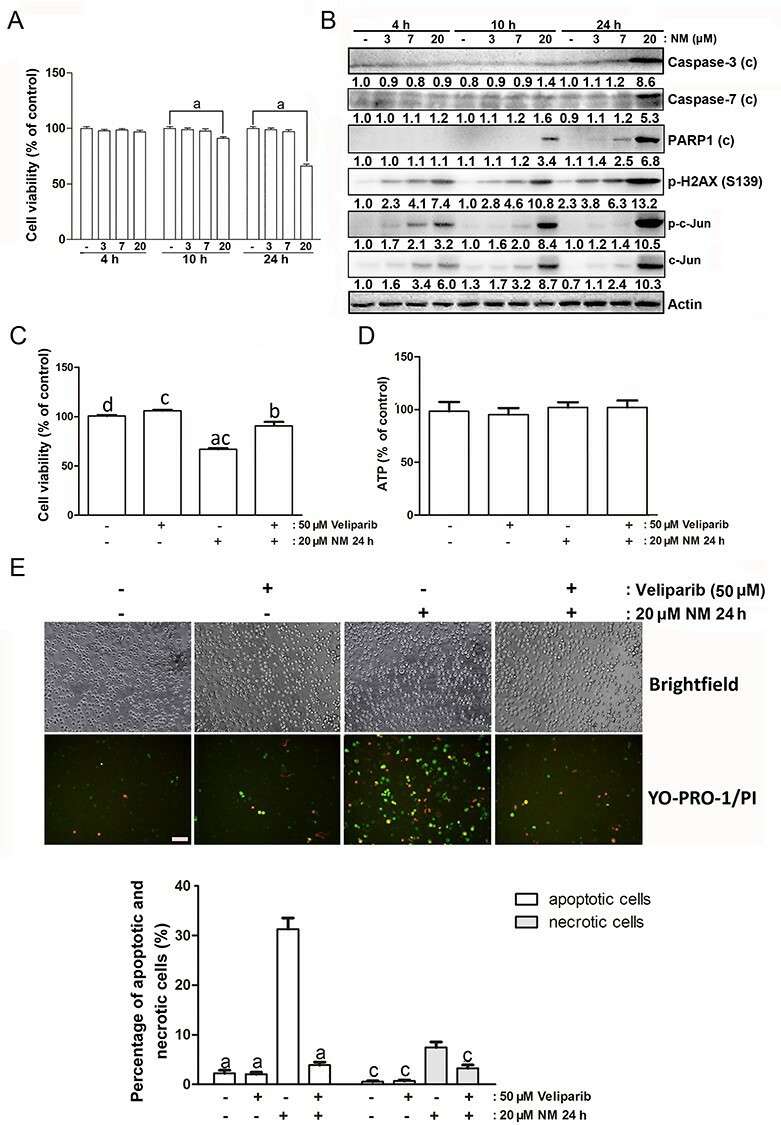 Fig. 1. The effect of PARP1 inhibitor on NM-caused cell death (Ye F, Dan G, et al., 2021).
Fig. 1. The effect of PARP1 inhibitor on NM-caused cell death (Ye F, Dan G, et al., 2021).
TRPV1 Activation is Crucial for Allergen-induced DUOX1 Activation and EGFR Signaling in Innate Airway Epithelial Responses
Airway epithelium triggers immune responses to airborne allergens through IL-33 release and subsequent activation of type 2 immune pathways. Although calcium-dependent signaling pathways participate in this process research has yet to fully clarify the functions of PAR2 receptors and TRP channels such as TRPV1. Schiffers et al. examined PAR2 and TRPV1 functions in IL-33 secretion from allergens using human nasal and mouse tracheal epithelial cells.
To further elucidate the importance of TRPV1 in allergen-induced responses, we performed complementary studies with immortalized human bronchial epithelial (HBE1) cells and mouse tracheal epithelial (MTE) cells. TRPV1 inhibitors decreased IL-33 secretion from HBE1 and MTE cells but TRPV1/TRPV4 agonists led to increased secretion (Fig. 2A and B). TRPV1 inhibition diminished both EGFR activation triggered by allergens and ATP in HBE1 and MTE cells (Fig. 2C–E) while siRNA-mediated TRPV1 silencing significantly decreased IL-33 and H2O2 production after allergen exposure and ATP and capsaicin stimulation (Fig. 2F and G). The TRPV4 selective nature of GSK1016790A remains evident as its effect on IL-33 was stable despite TRPV1 siRNA treatment (Fig. 2F). TRPV1 serves as an essential component for the activation of DUOX1 and EGFR by allergens and the secretion of IL-33.
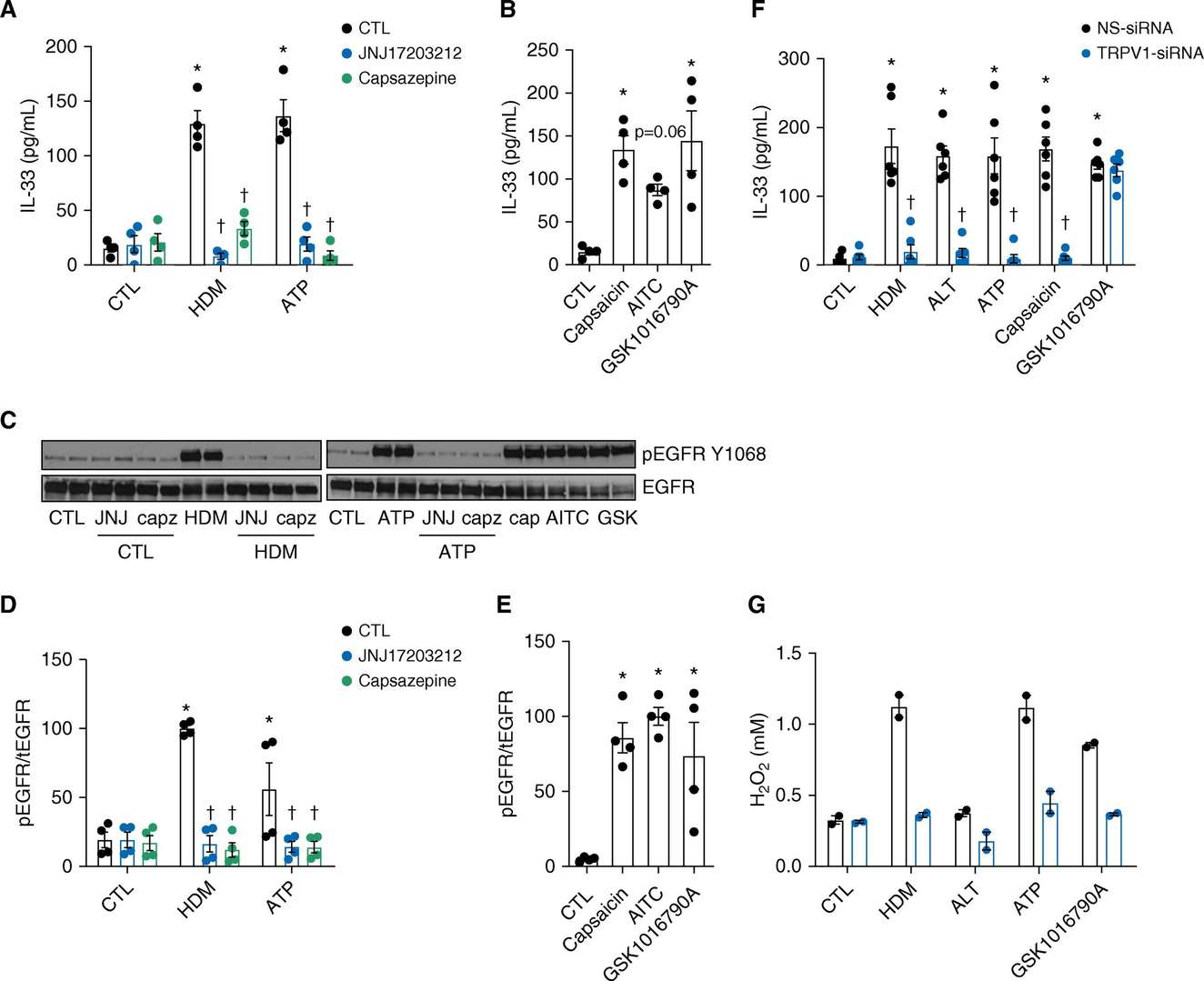 Fig. 2. Involvement of TRPV1 in allergen-induced innate epithelial responses (Schiffers C, Hristova M, et al., 2019).
Fig. 2. Involvement of TRPV1 in allergen-induced innate epithelial responses (Schiffers C, Hristova M, et al., 2019).
Ask a Question
Write your own review