Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
Farage
Cat.No.: CSC-C9100W
Species: Human
Source: lymph node
Morphology: lymphoblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The Farage cell line was adapted to cell culture in 1990. It was derived from a lymph node biopsy taken from a patient diagnosed with diffuse large cell non-Hodgkin's lymphoma (DLCL). This aggressive form of lymphoma is characterized by the proliferation of large, abnormal B lymphocytes that have lost their normal regulatory mechanisms.
The Farage cell line has also been used to create the Farage-Luc2 cell line, which can serve as a target cancer cell for in vitro killing assays by CD20 CAR-T cells, and is expected to work for CD19 CAR-T cells as well. The Farage-Luc2 cells express high levels of CD20. This makes the cell line an ideal model for in vitro studies of CD19-specific CAR-T cells due to its excellent signal/background ratio and stable luciferase expression.
The establishment of the Farage cell line provided researchers with a valuable tool for studying the biology and potential treatment approaches for this type of non-Hodgkin's lymphoma. As a cell line derived directly from a patient sample, the Farage cells maintain many of the key molecular and phenotypic features of the original tumor cells. This allows investigators to use the Farage model to better understand the genetic alterations, signaling pathways, and other factors that drive the uncontrolled growth and spread of DLCL.
Lack of Ig Polypeptide Chain Production in Farage Cells
To see whether Farage cells produce μ and Ig κ chains, the cells were metabolically labeled with 35S-methionine and the cell lysates were immune precipitated using anti-μ and aIIti-κ sera. Daudi and DG-75 B-lymphomas known to express surface IgM (κ) served as positive controls. Fig. 1 shows that in contrast to Daudi, radioactively labeled μ and κ chains could not be detected in Farage cytoplasmic cell lysates even following a long exposure time. In Daudi cells (Fig. 1) and DG-75 (not shown), large amounts of labeled μ chains were detected by either anti-μ or anti-κ reagents, indicating that both cell types produced μ and κ polypeptide chains. On the other hand, labeled κ was not detected at all in DG-75, and was detected at a low quantity in Daudi cells, where it was present in immune precipitates formed with anti-κ and not when the anti-μ agent was used (Fig. 1). Our results show that the κ light chains in Daudi, (and even more so in DG75 cells), are poorly labeled probably due to lack of the amino acid methionine in their κ light chains.
Because of the κ-chain labeling problem, immunoblots were done on the cytoplasmic lysates of DG-75 and Farage cells using enhanced chemiluminescence. Fig. 1B shows that whereas DG-75 contained both μ and κ polypeptide chains, no Ig chains could be detected in Farage cells. A band of a somewhat higher molecular size than the μ chain appeared in Farage cell lysates. However, a similar band is also present in the K562 negative control. To find out whether Ig chains were abnormally directed to the perinuclear or nuclear compartments of Farage cells, the nuclear pellet formed following NP40 cell lysis was lysed by SDS and deoxycholate and then immunoprecipitated with various antisera. Whereas μ chains could be detected in the "nuclear lysates" of Daudi cells by either anti-μ or anti-κ sera, no labeled Ig chains were detected in similar lysates made from Farage cells (not shown). Examination of the culture medium of Daudi, DG75, and Farage cells showed that labeled IgM was secreted by DG-75 but not by Farage or Daudi cells (not shown). The latter finding fits the classification of DG-75 as a sporadic (American) type BL, representing a more advanced stage of the B-cell differentiation pathway, as compared to Daudi cells classified as an endemic (African) type BL.
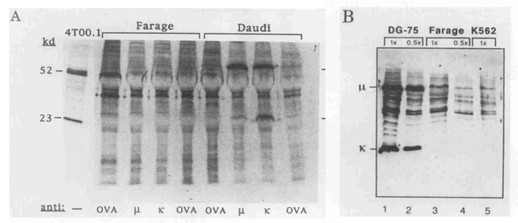 Fig. 1 Farage cells do not synthesize Ig-polypeptide chains. (Baruch M, et al., 1996)
Fig. 1 Farage cells do not synthesize Ig-polypeptide chains. (Baruch M, et al., 1996)
Generation of SLAMF1-Deficient Farage Cells
SLAMF1 was highly expressed in Farage cells, an EBV-positive B cell lymphoma cell line. To generate SLAMF1-deficient Farage cells, two sgRNAs targeting exon 3 of SLAMF1 were designed by using CRISPR/Cas9 technology (Fig. 2A). The two sgRNAs coupled with Cas9 expression vectors were transfected, and three cell lines named 3C, 8C, and 4E were obtained by serial dilution and mutational analysis (Fig. 2B). As shown in Fig. 2B, two bands were observed in 4E after PCR amplification, indicating a deletion of exon 3 in one allele (Fig. 2B). Sub-bands appeared after cleavage of heteroduplex in 3C, 8C and 4E, indicating the presence of at least one indel mutation (Fig. 2B). Whether the expression level of SLAMF1 was decreased at the cell surface was tested by using FACS analysis. SLAMF1 was almost negative in the three cell lines compared with SLAMF1 wild-type Farage cells (Fig. 2C). This result was further confirmed by western blot analysis with the anti-human SLAMF1 antibody, which was a different clone than that used for FACS analysis. SLAMF1 was not detected or remarkably decreased in mutant cell lines, whereas LMP1 had no discernable change between SLAMF1 wild-type and mutant cells (Fig. 2D).
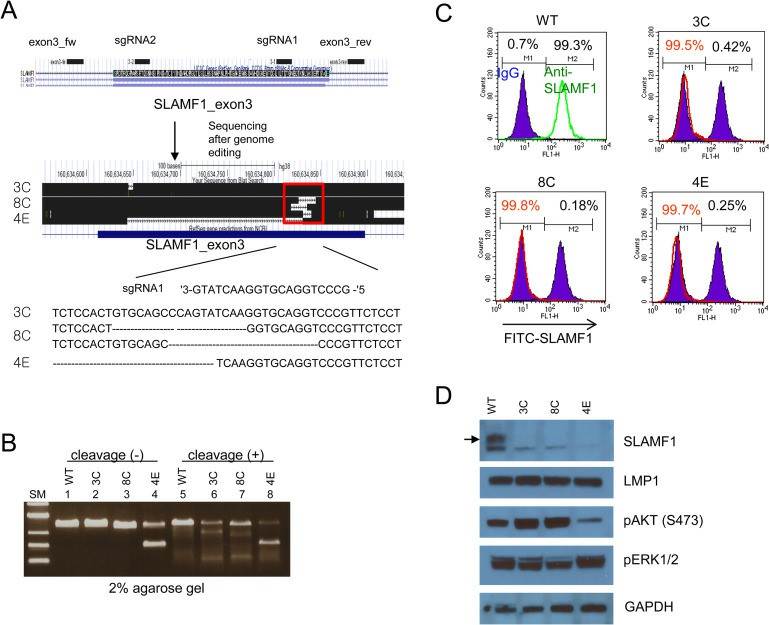 Fig. 2 SLAMF1-deficient Farage cells were cloned by using CRISPR/Cas9. (Yoon H, et al., 2020)
Fig. 2 SLAMF1-deficient Farage cells were cloned by using CRISPR/Cas9. (Yoon H, et al., 2020)
Challenges in establishing tumor cell lines include contamination issues, genetic instability, and the potential for the loss of original tumor characteristics during in vitro culturing.
The Farage cell line was established in 1990 from a lymph node biopsy of a patient diagnosed with diffuse large cell non-Hodgkin's lymphoma (DLCL).
The Farage cell line has been used to create the Farage-Luc2 cell line, which expresses high levels of CD20, making it an ideal model for in vitro studies of CD19-specific CAR-T cells due to its excellent signal/background ratio and stable luciferase expression.
The Farage cell line has a doubling time of approximately 48 hours.
Ask a Question
Average Rating: 4.7 | 3 Scientist has reviewed this product
Intuitive protocols
Even researchers with limited experience in cell culture techniques can work with these cells successfully, thanks to the intuitive protocols provided.
02 Dec 2023
Ease of use
After sales services
Value for money
High-quality products
The Farage cells we purchased from Creative Bioarray were of quality and have been instrumental in our lymphoma research.
21 May 2024
Ease of use
After sales services
Value for money
Excellent growth and performance
The Farage cells from Creative Bioarray have consistently shown excellent growth and performance.
17 Apr 2024
Ease of use
After sales services
Value for money
Write your own review