What Are Metabolism-Mediated Drug-Drug Interactions?
Therapeutics undergo metabolism as a major biotransformation pathway for their bioactivation or clearance from the body, which is a well-known factor affecting drug pharmacokinetics, safety, and efficacy. Drug metabolism primarily occurs in the liver and intestine, where a wide variety of drug-metabolizing enzymes, are generally divided into Phase I enzymes like cytochrome P450s (CYP450s) and Phase II enzymes as UDP glucuronosyltransferases (UGTs). When two or more drugs are co-administered, their metabolic pathways can intersect, leading to a phenomenon known as metabolism-mediated drug-drug interactions (MMDDIs).
MMDDIs occur when the metabolism of one drug is altered by the presence of another, resulting in changes in the systemic exposure and/or therapeutic response of the affected drug. These interactions can have significant clinical implications, potentially leading to adverse events, therapeutic failure, or even life-threatening consequences.
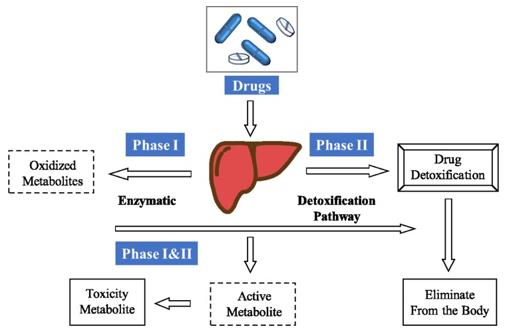 Fig.1 General pathway of drug metabolism. (Wang D, et al., 2020)
Fig.1 General pathway of drug metabolism. (Wang D, et al., 2020)
Mechanisms of MMDDIs
At the core of MMDDIs lies the intricate interplay between drug molecules and the metabolic enzymes responsible for their biotransformation. The primary mechanisms by which these interactions occur can be categorized as follows:
Enzyme Inhibition
One drug can inhibit the activity of the metabolic enzymes responsible for the biotransformation of another drug, leading to increased systemic exposure and potential toxicity of the affected drug. This inhibition can be competitive, non-competitive, or irreversible, depending on the specific interaction.
| Inhibition types | Details |
| Competitive inhibition | The two drugs compete for the same active site on the metabolic enzyme, reducing the enzyme's availability for the affected drug. |
| Non-competitive inhibition | The inhibitor binds to a site other than the active site, causing a conformational change that reduces the enzyme's activity. |
| Irreversible inhibition | The inhibitor binds covalently to the enzyme, permanently inactivating it. |
Enzyme Induction
Another mechanism of MMDDIs is enzyme induction, where one drug induces the expression or activity of the metabolic enzymes responsible for the biotransformation of another drug. This can result in increased metabolism and decreased systemic exposure of the affected drug, potentially leading to reduced therapeutic efficacy or the need for dosage adjustments. Enzyme induction typically involves the activation of nuclear receptors, such as the pregnane X receptor (PXR) or the constitutive androstane receptor (CAR), which then upregulate the expression of specific metabolic enzymes.
Substrate Competition
In some cases, MMDDIs can occur due to substrate competition, where two or more drugs compete for the same metabolic enzymes. This can lead to altered pharmacokinetics and potential changes in the efficacy or safety profiles of the involved drugs.
For instance, the anti-HIV drug ritonavir is a potent inhibitor of CYP3A4, the enzyme responsible for the metabolism of many drugs. When ritonavir is co-administered with other CYP3A4 substrates, such as certain immunosuppressants or antifungals, the resulting competition can lead to increased systemic exposure and potential toxicity of the affected drugs.
Metabolic Pathways Involved in Drug-Drug Interactions
Cytochrome P450 Enzymes
The cytochrome P450 (CYP) enzyme system is undoubtedly the most extensively studied and well-documented contributor to metabolism-mediated DDIs. This family of enzymes, particularly CYP3A4, CYP2D6, CYP2C9, and CYP2C19, are responsible for the biotransformation of a vast array of pharmaceutical compounds.
When two or more drugs compete for the same CYP enzymes, it can result in altered drug concentrations and potentially lead to either increased or decreased therapeutic effects. For example, if Drug A is a substrate for CYP3A4 and Drug B is an inhibitor of this enzyme, the metabolism of Drug A may be impaired, leading to a higher concentration of the active drug in the body. Conversely, if Drug A is a substrate and Drug B is an inducer of CYP3A4, the enhanced metabolism of Drug A can result in subtherapeutic levels, potentially compromising its efficacy.
Phase II Metabolic Enzymes
In addition to the CYP system, phase II metabolic enzymes, such as UDP-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs), also play a crucial role in the biotransformation and elimination of drugs. These enzymes catalyze the conjugation of drugs and their metabolites with endogenous molecules, facilitating their excretion.
Interactions involving phase II enzymes can have significant implications for drug pharmacokinetics. For instance, if Drug A is a substrate for a particular UGT enzyme and Drug B inhibits the activity of that enzyme, the clearance of Drug A may be reduced, leading to elevated plasma concentrations and an increased risk of adverse effects.
Drug Transporters
The movement of drugs and their metabolites across cellular membranes is facilitated by various drug transporter proteins, such as P-glycoprotein (P-gp) and organic anion-transporting polypeptides (OATPs). These transporters can influence the absorption, distribution, and elimination of medications, and their modulation by other drugs can result in significant DDIs.
Approaches to Evaluating MMDDIs
In Vitro Approaches to Evaluating MMDDIs
By using human-derived cell lines, liver microsomes, and recombinant enzymes, researchers can gain valuable insights into the underlying mechanisms of MMDDIs.
- Enzyme kinetic studies. Evaluate the type and extent of enzyme inhibition and determine the inhibition constants that quantify the potency of the interaction. Assess the ability of a drug to induce the expression or activity of metabolic enzymes, including the magnitude and time course of the induction response.
- High-throughput screening. HTS platforms allow for the rapid assessment of a large number of drug candidates for their potential to inhibit or induce metabolic enzymes. This screening process helps identify promising drug candidates and prioritize them for further in-depth investigations.
In Vivo Approaches to Evaluating MMDDIs
Animal models, such as rodents and non-human primates, are employed to investigate the impact of MMDDIs on pharmacokinetics and pharmacodynamics in a more physiologically relevant setting.
- Pharmacokinetic interactions. Evaluate the effect of one drug on the absorption, distribution, metabolism, and elimination of another drug, providing insights into the potential for clinically relevant interactions.
- Pharmacodynamic interactions. Assess the impact of MMDDIs on the intended therapeutic effects or adverse events of the affected drug, allowing for the evaluation of the clinical significance of the interaction.
Creative Bioarray Relevant Recommendations
| Service Types | Description |
| Cytochrome P450 Induction Assay | CYP450 enzymes play an essential role in drug metabolism. Creative Bioarray aims to help our clients with evaluating the interaction potential of your compounds. |
| Cytochrome P450 Inhibition Assay | The inhibition of CYPs is associated with an increase in the incidence of clinical drug-drug interactions (DDI). CYP450 inhibition data can be used to design strategies to study clinical DDI studies. Creative Bioarray aims to help our clients with evaluating the interaction potential of your compounds. |
| UGT Induction | Of all the isoforms, UGT1A1, UGT1A9, and UGT2B7 are available for induction assays. Creative Bioarray is providing these assays for your drug development needs. |
| UGT Inhibition | Creative Bioarray provides customers with the UDP-glucuronosyltransferases (UGTs) enzyme inhibition test to help study the metabolic pathways of the tested drugs except for CYPs. |
Reference
- Wang D, et al. (2020). "Deep Learning Based Drug Metabolites Prediction." Front Pharmacol. 10: 1586.

