What Are Compartment Models in Pharmacokinetics?
Pharmacokinetic (PK) modeling is a tool used to help drug developers understand a drug's effects on the body by analyzing its absorption, distribution, metabolism, and excretion (ADME) properties. These effects are typically summarized using PK parameters such as clearance and volume of distribution which are necessary for understanding the effects of a drug on the body. PK models can range in complexity from models with a single compartment to models containing hundreds of compartments. Each type of model from the simplest, one-compartment model to more complex models has its applications and can be used to gain valuable information from clinical or non-clinical data.
What Is a Compartmental Model?
Compartmental modeling is a model-based method used for estimating PK parameters. To apply this method, the body is divided up into hypothetical compartments. Often, the containers used in compartmental modeling do not represent actual physiological tissues in the body but are used as a proxy so that PK parameters can be determined. For example, in compartmental modeling, the rates of absorption and/or clearance can be modified between compartments to model the effect of such changes, representing a disease state or induction of metabolism. Compartmental modeling is a general term that refers to a model with at least one compartment. Typical compartmental models have between one and three compartments, but more compartments can be added to the model depending on the application. For example, in a type of compartmental modeling known as whole-body physiologically based pharmacokinetic (PBPK) modeling, there can be as many as one compartment for each organ of the body.
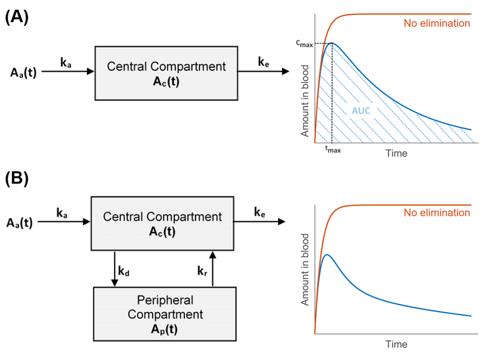 Fig.1 Schematic representation of 1-compartment (A) and 2-compartment (B) pharmacokinetic models. (Silva JS, et al., 2021)
Fig.1 Schematic representation of 1-compartment (A) and 2-compartment (B) pharmacokinetic models. (Silva JS, et al., 2021)
Types of Compartment Models
| Model types | Details |
| One-compartment model | The one-compartment model is the simplest representation of drug behavior, assuming the drug is distributed uniformly throughout a single, well-mixed compartment, typically representing the central (blood) compartment. This model is often used for drugs with rapid distribution and elimination kinetics, such as many antibiotics and anesthetic agents. |
| Two-compartment model | The two-compartment model introduces an additional peripheral compartment, typically representing tissues and organs, to better capture the complex distribution and elimination processes of drugs. This model is more suitable for drugs with slower distribution and/or elimination kinetics, such as many cardiovascular and antidepressant medications. |
| Multi-compartment model | For drugs with even more complex pharmacokinetic behavior, multi-compartment models can be employed, incorporating additional compartments to represent various physiological spaces (e.g., liver, kidneys, brain) and distribution processes. These advanced models are particularly useful for understanding the PK of drugs with extensive tissue binding or complex elimination pathways. |
Pharmacokinetic Parameters in Compartment Models
Compartment models rely on several key pharmacokinetic parameters to characterize drug behavior:
| PK parameters | Details |
| Absorption rate constant (Ka) | It describes the rate at which the drug is absorbed from the site of administration into the central compartment. A higher Ka value indicates a faster absorption rate, while a lower Ka value suggests a slower absorption process. |
| Elimination rate constant (Ke) | It represents the rate at which the drug is eliminated from the body, typically through metabolism and/or excretion. A higher Ke value indicates a faster elimination rate, meaning the drug is cleared from the body more rapidly, while a lower Ke value corresponds to a slower elimination process. |
| Volume of distribution (Vd) | It reflects the apparent volume in which the drug is distributed, providing insight into the drug's tissue distribution and binding. A larger Vd generally indicates that the drug is more widely distributed in the body, while a smaller Vd suggests a more limited distribution. |
| Clearance (CL) | It is a measure of the volume of blood or plasma that is completely cleared of the drug per unit of time. It represents the body's ability to remove the drug from the systemic circulation, through processes such as metabolism, excretion, or a combination of both. |
| Half-life (t1/2) | It represents the time it takes for the drug concentration to decrease by 50% and is a crucial parameter for determining dosing regimens. It is directly related to the Ke and reflects the time it takes for the drug to be cleared from the body. |
Applications of Compartment Models in PK
- Drug concentration and exposure time. Compartment models enable researchers and clinicians to accurately predict the concentrations of drugs in the body over time. By inputting the relevant pharmacokinetic parameters, such as Ka, Ke, and Vd, these models can generate detailed profiles of how drug levels fluctuate throughout treatment.
- Dose optimization and personalized dosing. Building upon the ability to predict drug concentrations, compartment models play a crucial role in optimizing drug dosing and tailoring therapies to individual patient characteristics. By incorporating factors like age, body weight, organ function, and genetic variations, these models can help determine the appropriate dose and dosing schedule for each patient.
- Understanding drug distribution and elimination. Compartment models provide valuable insights into the complex processes of drug distribution, metabolism, and elimination, guiding the development of new therapeutic agents and formulations. This knowledge is instrumental in the development of new drugs, as it allows for the rational design of molecular structures and formulations that optimize the desired pharmacokinetic profile.
Creative Bioarray Relevant Recommendations
| Service Types | Description |
| Pharmacokinetic and Toxicokinetic Studies | Creative Bioarray provides professional PK/TK testing services to help our customers choose pharmaceutical compounds and effective and safe dosing regimens. |
| PK/PD Biomarker Analysis | Creative Bioarray's bioanalytical laboratories can develop and validate methods for analyzing biomarkers in preclinical and clinical samples such as plasma, serum, urine, tissue, and cerebrospinal fluid. We provide assays depending on your development phase and purpose regarding the biomarker. |
Reference
- Silva JS, et al. (2021). "Towards the development of delivery systems of bioactive compounds with eyes set on pharmacokinetics." Modeling and Control of Drug Delivery Systems. 125-144.

