Pharmacokinetics of Therapeutic Peptides
Therapeutic peptides (short chains of amino acids) are a class of drugs that have recently been embraced for their widespread biological use and medical potential. Peptide medicine first got a patent in the early 20th century, when insulin was isolated and used as a medical drug. Since then, peptides have transitioned from natural hormones to sophisticated synthetic biomolecules that are adapted for physiological use. One of the most significant advances in the peptide drug pipeline was solid-phase peptide synthesis (SPPS) developed in the 1960s that enabled high-efficiency production of highly complex peptides. Artificial and analytical technologies from recombinant DNA to high-performance liquid chromatography had pushed therapeutic peptides even further through the decades. Peptides have therapeutic applications in endocrinology, oncology, cardiovascular and other therapies; more than 80 peptide medications have been approved worldwide, with many more on the way.
Advantages and Disadvantages of Therapeutic Peptides
Advantages
Therapeutic peptides offer several advantages over traditional small molecules and biologics, primarily because of their biological adequacy and affinities. They mimic natural physiology by adhering to receptors at the surface of cells with a high degree of affinity and specificity, and so are less immunogenic and have fewer adverse effects. In addition, they provide a good safety profile and are relatively safe and toxic in nature, suitable for long-term use.
Peptides offer further unique possibilities for modification and improvement. New technologies in peptide engineering gave us the potential to produce peptide-drug conjugates, which blend the high specificity of peptides with the potent therapeutic properties of small molecules. This has resulted in increased cellular absorbance, longer plasma half-life, and improved therapeutic effect.
Disadvantages
For all their benefits, therapeutic peptides are quite challenging to pharmacokinetically manage. Their primary disadvantages are membrane inpermeability and low in vivo stability. Because peptides are larger and hydrophilic than small molecules, they cannot typically cross cell membranes to access intracellular targets, and thus cannot be useful for therapeutic purposes.
Secondly, peptides can be easily broken down by proteolytic enzymes and have a very short half-life, requiring frequent dosing. Clycling, PEGylation and lipidation have been used to stabilize peptides and prolong half-life, although they can reduce biological function.
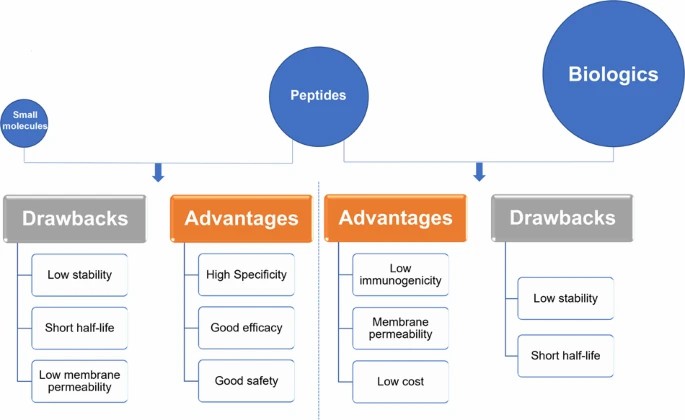 Fig. 1. Peptides versus small molecules and biologics. Comparison of advantages and drawbacks between peptides and small molecules or biologics (Muttenthaler M, King GF, et al., 2021).
Fig. 1. Peptides versus small molecules and biologics. Comparison of advantages and drawbacks between peptides and small molecules or biologics (Muttenthaler M, King GF, et al., 2021).
The Pharmacokinetics of Therapeutic Peptides
Absorption
- Parenteral Administration
The absorption of therapeutic peptides is primarily achieved through parenteral routes, including intravenous (IV), subcutaneous (SC), and intramuscular (IM) injections. IV provides the benefit of full systemic availability, avoiding digestive and liver breakdown, while enabling exact dosing control.
But SC and IM routes, while slightly slower because they could degrade at the injection site, provide practical advantages such as self-administration. They take up absorption through both capillary and lymphatic channels, and molecular size determines which is the most common absorption channel.
- Non-Invasive Routes
An effort to make peptide therapies more convenient and effective has also prompted research into non-invasive delivery modes like oral, buccal and transdermal. The bioavailability through oral administration is restricted by harsh gastrointestinal environment and poor mucosal permeability, but new formulations such as permeation enhancers and encapsulation have some promising answers.
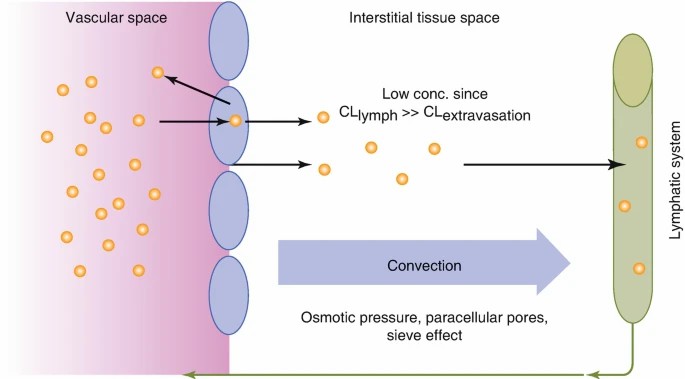 Fig. 2. Distribution mechanisms of therapeutic proteins: convective extravasation rather than diffusion as major distribution process (Meibohm B, 2019).
Fig. 2. Distribution mechanisms of therapeutic proteins: convective extravasation rather than diffusion as major distribution process (Meibohm B, 2019).
Distribution
Following absorption, peptides are distributed primarily within the extracellular space due to their size and hydrophilicity. Their volume of distribution is generally limited, with a biexponential plasma concentration-time profile typical for peptides. Peptide distribution is further influenced by binding to endogenous proteins, which can significantly impact their pharmacokinetic profiles and therapeutic efficacy.
Metabolism
Peptide metabolism is oxidized by proteolytic reaction, generally occurring in the liver, kidneys and other tissues. Peptidases and proteases break down peptides into amino acids, and those are reabsorbed into the body's amino acid supply.
Metabolic stability is a major concern, and it is often used as an antioxidant, cyclized, PEGylated or in combination with D-amino acids for resistance to proteolytic enzymes. These changes are designed to prolong the half-life of the peptide, increase its bioavailability, and ultimately enhance therapeutic performance.
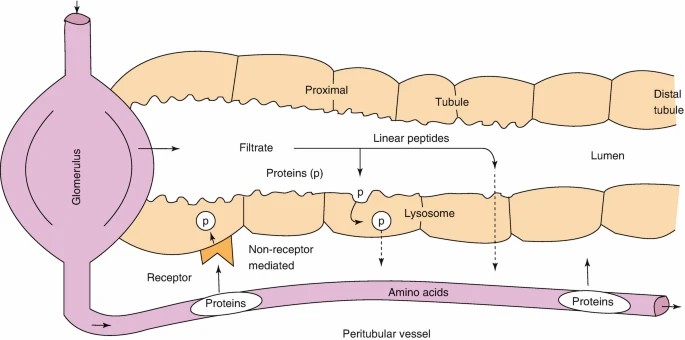 Fig. 3. Pathways of renal metabolism of peptides and proteins: glomerular filtration followed by either (1) intraluminal metabolism or (2) tubular reabsorption with intracellular lysosomal metabolism and (3) peritubular extraction with intracellular lysosomal metabolism (Meibohm B, 2019).
Fig. 3. Pathways of renal metabolism of peptides and proteins: glomerular filtration followed by either (1) intraluminal metabolism or (2) tubular reabsorption with intracellular lysosomal metabolism and (3) peritubular extraction with intracellular lysosomal metabolism (Meibohm B, 2019).
Elimination
Renal excretion is the principal elimination route for therapeutic peptides, particularly those with molecular weights below the renal filtration threshold. The peptides go through glomerular filtering and then intraluminal metabolism, where they're hydrolyzed by brush border enzymes in the tubules of the kidneys.
For larger peptides, biliary excretion and subsequent fecal elimination may play a role, though this pathway is less common. The rapid elimination of peptides poses a challenge, often requiring innovative formulation strategies to sustain therapeutic concentrations over time.
Conclusion
Therapeutic peptide pharmacokinetics involves an intricate set of processes – absorption, distribution, metabolism, and elimination. The intrinsic issues surrounding peptide delivery and stability must be resolved before the therapy can be truly enhanced. Peptide engineering, along with novel delivery systems, has the potential to transform peptide treatments into more efficient and cost-effective therapies that could drive the pharmaceutical revolution ahead.
| Products & Services | Description |
| Quantitative Tissue Distribution | Creative Bioarray provides quantitative tissue distribution services to help our customers visualize true tissue distribution, facilitate tissue PK analysis and dosimetry prediction before the initiation of human mass balance studies. |
| In Vitro Metabolism | With extensive knowledge and experience in drug discovery, Creative Bioarray can provide standard, cost-effective in vitro metabolism services, including drug metabolic stability services and drug-drug interaction services to support your drug development process. |
| Pharmacokinetic and Toxicokinetic Studies | Creative Bioarray provides professional PK/TK testing services to help our customers choose pharmaceutical compounds and effective and safe dosing regimens. |
References
- Muttenthaler M, King GF, et al. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021. 20(4):309-325.
- Meibohm B. Pharmacokinetics and Pharmacodynamics of Therapeutic Peptides and Proteins. Pharmaceutical Biotechnology. 2019. 105-137.
- Diao L, Meibohm B. Pharmacokinetics and Pharmacokinetic–Pharmacodynamic Correlations of Therapeutic Peptides. Clin Pharmacokinet. 2013. 52, 855–868.

