Pharmacokinetics Considerations for Antibody Drug Conjugates
Antibody-drug conjugates (ADCs) represent a significant advancement in targeted cancer therapy, merging the specificity of monoclonal antibodies with the potency of cytotoxic drugs. Understanding the pharmacokinetics (PK) of ADCs is crucial for optimizing their therapeutic efficacy while minimizing systemic toxicity.
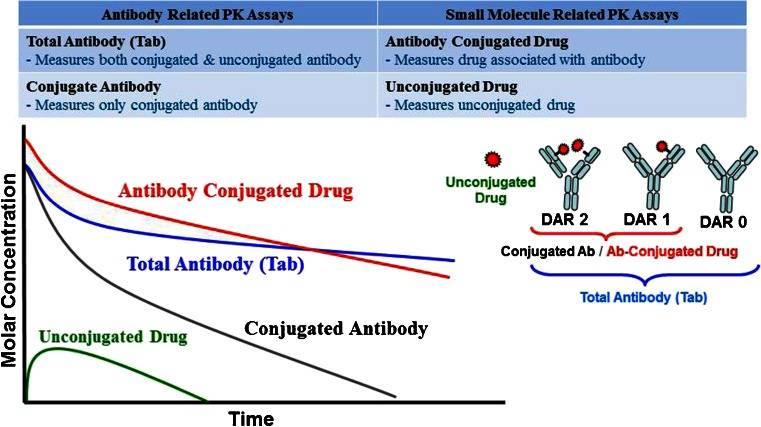 Fig. 1 Pharmacokinetic profiles of different analytes. (Kamath AV and Iyer S, 2015)
Fig. 1 Pharmacokinetic profiles of different analytes. (Kamath AV and Iyer S, 2015)
What Are Antibody Drug Conjugates?
ADCs are a class of biopharmaceutical agents that combine the targeting ability of monoclonal antibodies with the cytotoxic potential of a drug payload. These hybrid molecules work by selectively binding to antigenic targets on the surface of diseased cells, followed by internalization and release of the cytotoxic drug, leading to cell death. The components of an ADC include the antibody, a linker, and the cytotoxic drug, each playing a critical role in the overall pharmacokinetic behavior of the conjugate.
The antibody component of an ADC is responsible for its specificity and longevity in circulation. The linker serves as the bridge between the antibody and the drug payload, determining the stability and site of drug release. The cytotoxic drug, often a highly potent compound, is the effector molecule responsible for inducing cell death.
Influence of ADC Characteristics on its Pharmacokinetics
Antibody
The choice of antibody significantly affects the PK of the ADC. Monoclonal antibodies exhibit varying half-lives, typically ranging from days to weeks, influenced by their structure, isotype, and the presence of glycosylation patterns. For instance, IgG1 antibodies often demonstrate prolonged circulation due to their ability to engage with the neonatal Fc receptor (FcRn), which recycles antibodies back into the bloodstream. The affinity of the antibody for its target antigen also influences PK; higher affinity can lead to increased uptake by target cells, while potentially reducing systemic exposure.
Linker
The linker plays a pivotal role in the stability and release kinetics of the ADC. A stable linker ensures that the cytotoxic drug remains attached during circulation, preventing premature release and systemic toxicity. Linkers can be classified into cleavable and non-cleavable types. Cleavable linkers, such as those sensitive to pH or specific enzymes, allow for drug release in the acidic environment of endosomes, enhancing tumor specificity. In contrast, non-cleavable linkers may provide more stability during circulation but can lead to higher systemic exposure if the ADC is not efficiently taken up by the target cells.
Site of conjugation
The site of conjugation on the antibody can also influence PK. Conjugation at specific amino acid residues, such as lysine or cysteine, can alter the antibody's structural integrity and its interaction with biological systems. For example, conjugation at the Fc region may enhance binding to FcRn, extending circulation time, while conjugation at the Fab region could improve binding affinity to the target antigen. The distribution and clearance of the ADC can be affected by these modifications, impacting overall therapeutic efficacy.
Drug load (Drug-to-antibody ratio, DAR)
The drug-to-antibody ratio (DAR) is a critical parameter that influences the PK of ADCs. Higher DARs can enhance cytotoxicity but may also result in increased immunogenicity and altered pharmacokinetic profiles. Finding an optimal DAR is essential to maximize therapeutic effects while minimizing adverse effects. Studies have shown that ADCs with a DAR of 3-4 often strike a balance between potency and safety, providing a favorable PK profile.
Cytotoxic drug
The choice of cytotoxic drug significantly affects the overall PK of the ADC. Different drugs exhibit distinct solubility, metabolism, and clearance characteristics. For example, maytansinoids and auristatins, commonly used in ADC formulations, have different mechanisms of action and PK properties. The inherent properties of these cytotoxic agents, including their half-lives and tissue distribution, will ultimately influence the ADC's efficacy and safety profile.
Preclinical PK Strategies to Evaluate ADCs
Evaluating the PK of ADCs during the preclinical phase is essential for understanding their behavior in vivo and predicting their performance in clinical settings. A robust preclinical PK strategy helps in identifying optimal dosing regimens, potential toxicity, and overall therapeutic efficacy.
Bioanalysis research
Accurate measurement of ADC concentrations in biological samples is crucial. This involves developing sensitive and specific bioanalytical methods to quantify ADCs in plasma, tissues, and other biological fluids. Common techniques include:
- Enzyme-linked immunosorbent assay (ELISA): Highly sensitive for detecting ADCs, especially for assessing the intact conjugate.
- Liquid chromatography-mass spectrometry (LC-MS/MS): Provides detailed information about the ADC and its metabolites, allowing for the quantification of both the antibody and the cytotoxic drug.
Dosing studies
Different dosing regimens (e.g., single vs. multiple dosing) are evaluated to understand the impact on PK profiles and therapeutic outcomes.
Tissue distribution studies
Tissue distribution studies are critical for understanding how an ADC disperses throughout the body and its potential for off-target effects. Biodistribution studies often involve administering radiolabeled ADCs to animal models and assessing the distribution of radioactivity in various tissues over time. The immunohistochemistry technique allows for the visualization of ADC localization in tissues, providing insights into target engagement and potential toxicity.
Metabolic studies
Understanding the metabolic pathways of ADC components is essential for predicting their behavior and interactions in the body. Evaluating how long the ADC remains intact in circulation before being metabolized or cleared. Identifying potential interactions with metabolic enzymes can help predict drug-drug interactions that may impact efficacy or safety.
Creative Bioarray Relevant Recommendations
| Service Types | Description |
| Pharmacokinetic and Toxicokinetic Studies | Creative Bioarray provides professional PK/TK testing services to help our customers choose pharmaceutical compounds and effective and safe dosing regimens. |
| PK/PD Biomarker Analysis | Creative Bioarray's bioanalytical laboratories can develop and validate methods for analyzing biomarkers in preclinical and clinical samples such as plasma, serum, urine, tissue, and cerebrospinal fluid. We provide assays depending on your development phase and purpose regarding the biomarker. |
Reference
- Kamath AV, Iyer S. (2015). Preclinical Pharmacokinetic Considerations for the Development of Antibody Drug Conjugates. Pharm Res. 32 (11): 3470-9.

