Organ-on-a-Chip Systems for Drug Screening
The limitations of existing drug screening technologies call for paradigm-breaking technologies like organ-on-a-chip (OOAC) systems. Traditional in vitro 2D cell cultures and animal models have been used for drug research for years. But such approaches cannot reliably identify human drug sensitivity because of inherent physiological variation among species and the simplistic nature of cell cultures. Animal testing, for instance, may not reflect the essential human metabolic pathways, making predictions about drug effectiveness and toxicity less accurate.
More recently, improvements in microfabrication and tissue engineering have made it possible to build OOAC systems capable of replicating human organs and offering a better representation of human physiology. This is particularly relevant considering that clinical trials are notoriously unsuccessful, due to unanticipated cardiotoxicity, hepatotoxicity and other side-effects not evident in early testing. Therefore, OOAC systems are becoming indispensable in pre-clinical drug screening, providing an intermediate platform between cellular and whole-organ analysis.
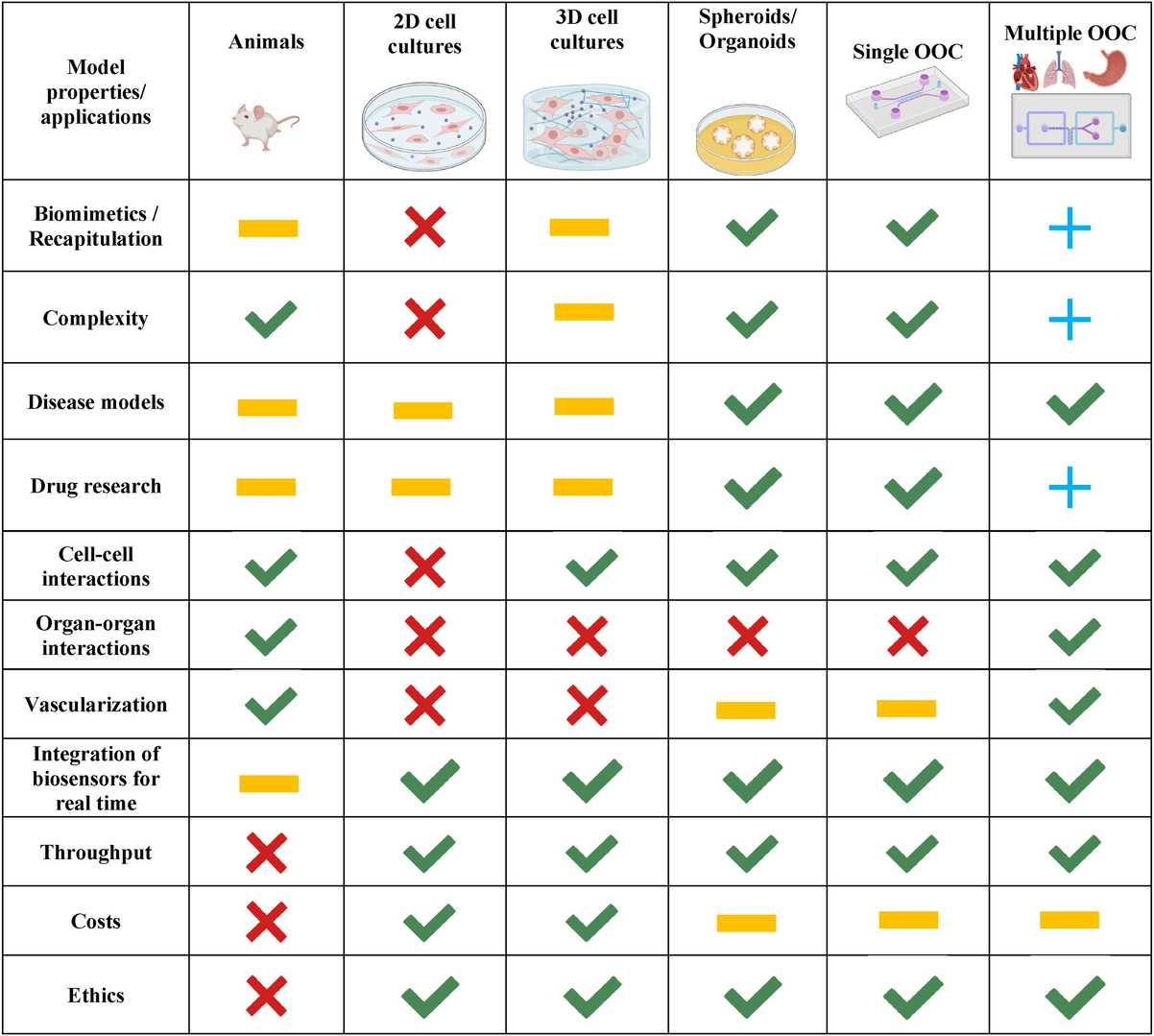 Fig. 1. Advantages of single and multiple OOAC platforms in comparison to other methodologies (Monteduro AG, Rizzato S, et al., 2023).
Fig. 1. Advantages of single and multiple OOAC platforms in comparison to other methodologies (Monteduro AG, Rizzato S, et al., 2023).
The Advantages of Organ-on-a-Chip Systems for Drug Screening
OOAC devices employ innovative technologies, such as microfluidics, biomaterials and stem-cell biology, to build platforms that emulate the complexity and function of human organs. These mechanisms offer several advantages over manual processes:
- Higher Predictive Accuracy: Because OOAC models are based on human cells, they eliminate cross-species differences and provide more human-relevant information. That improves prediction of drug effects – thereby minimizing late-stage failures.
- Organ Interactions: Multi-organ-on-a-chip systems allow organ-organ interactions that replicate almost perfectly the systemic architecture of human bodies. This enables a better understanding of pharmacokinetics and pharmacodynamics in multiple systems and provides information on drug metabolism, distribution and systemic toxicology.
- Ethical and Financial Advantages: Lowering the need for animal testing reduces ethical issues and the cost of maintaining animal models. Moreover, OOAC allows high-throughput screening, thus saving resources.
- Continuous Monitoring and Customization: Microsensors with real-time monitoring of physiological changes guarantee constant control over the environment of the experiment. Further, OOAC platforms can be personalized to meet the needs of individual patients, opening the way to personalized medicine.
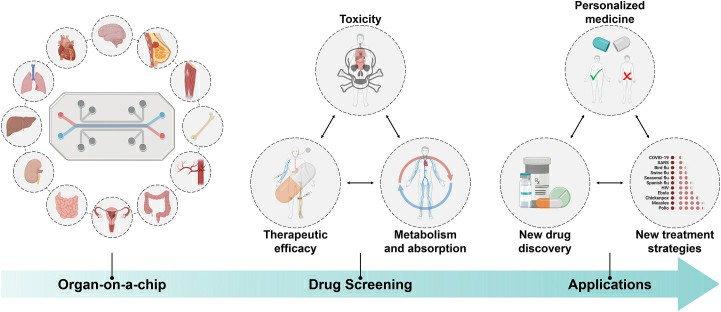 Fig. 2. Scheme of organ-on-a-chip for drug screening (Wang Y, Gao Y, et al., 2023).
Fig. 2. Scheme of organ-on-a-chip for drug screening (Wang Y, Gao Y, et al., 2023).
Drug Screening Based on Organ-on-a-Chip Platforms
Since OOAC technology was established and refined, advances in the drug screening of these systems for different organ models have been made:
- Heart-on-a-Chip: The heart-on-a-chip (heart-OAC) models simulate cardiac tissue-based physiology, critical to determining drug-induced cardiotoxicity. Since the heart is a central conduit of circulation, cardiac models use CMs, fibroblasts and endothelial cells to mimic human heart responses.
- Liver-on-a-Chip: Liver-OAC platforms are focusing on the liver's function as a detoxification and metabolism center. These networks involve hepatocytes and other liver cells, which can be used to identify hepatotoxicity and metabolic changes of medications. By re-modelling the hepatic microenvironment, liver-OACs can anticipate drug metabolism (as seen in the experiments with acetaminophen). These platforms improve the sensitivity of drug-induced liver damage and enable the assessment of metabolic byproducts.
- Kidney-on-a-Chip: The kidney-on-a-chip (kidney-OAC) machines are also used for nephrotoxicity studies because the kidney is such an important excretory and filtration organ. These chips mimic renal morphology and function, making it possible to evaluate drugs' effect on renal health. In the case of cyclosporine A, for instance, kidney-OAC has been successfully used to characterize its renal toxicant potential.
- Brain-on-a-Chip: Brain-on-a-chip platforms are the latest generation that take on the challenge of neurological drug discovery. They also enable them to mimic neural network and blood-brain barrier processes critical to CNS drug screening. This means that brain-OACs could be useful tools to investigate neurotoxicity and CNS drug delivery, particularly in disease-modifying conditions such as Alzheimer's.
- Other single-organ-on-a-chip: Other single-organ-on-a-chip include the lungs, intestines, skin and blood vessels. An alveolus chip, for instance, was constructed from co-culturing human alveolar epithelium, microvascular endothelium and infiltrating immune cells in an attempt to mimic the functions of the alveolar-capillary barrier. The intestine chip was designed to simulate the intestinal microenvironment via Caco-2 cells, porous nitrocellulose membranes and continuous flow collagen I. Furthermore, an inflammatory skin chip model was constructed by co-culturing human keratinocytes (HaCaTs), fibroblasts (HS27) and human umbilical vein endothelial cells (HUVECs) into different layers (epidermal, dermal and vascular layers) and perfusing with TNF-α to induce inflammation.
- Multi-Organ-on-a-Chip: Multi-OOACs combine multiple organ models on a microfluidic device for inter-organ interactions and holistic drug pharmacokinetics. By linking up models from liver, heart, intestine and kidney, such platforms replicate the complex physiology of the human body, providing information about drug candidates' pharmacokinetics and pharmacodynamics. Such combined systems enable accurate forecasting of systemic effects and toxicities, powerful preclinical test tools, and better drug development assurance.
Potential Applications of Organ-on-a-Chip in Drug Screening
Such micro-engineered systems are increasingly being used to predict human efficacy, toxicity and pharmacokinetics of drugs, opening up opportunities for novel phenotypic screening.
Efficacy assessment
The clinical success of drug candidates is often impaired by the fact that conventional models do not realistically simulate the human body. Organ-on-a-chip systems provide a game-changing approach, replicating intricate tissue signals and drug interactions.
Toxicity screening
Organ-on-a-chip platforms offer a sophisticated way to identify toxicities before they become clinically evident. Metabolomic measurements of metabolites in micro-engineered liver models have already been successfully applied to discover biomarkers of toxic action. Further, incorporated multi-organ systems mimic drug interactions across tissues to provide insight into compound-specific nephrotoxic and cardiotoxic effects, further enhancing drug candidates' safety.
Pharmacokinetics and multi-organ integration
Organism-on-chip technology has now also been used to simulate key pharmacokinetic events, including absorption and clearance. Liver-on-a-chips, often paired with computational models, have correctly predicted drug metabolism and clearance levels based on clinical evidence. These efforts now concentrate on creating interconnected multi-organ systems that recreate systemic pharmacokinetic and pharmacodynamic interactions. Such 'body-on-a-chip' simulations could reshape preclinical testing by providing a whole-body perspective on drug behavior in the body.
Phenotypic screening
The resurgence of phenotypic drug discovery illustrates the promise of organ-on-a-chip platforms for detecting sophisticated physiological behavior. Such systems provide a high-throughput, inexpensive replacement for classical models that can test drug repositioning strategies. A good example is the liver cancer-on-a-chip to study artemisinin's anticancer activity, showing how organ-on-a-chip can uncover novel therapeutic uses for already developed medicines.
References
- Wang Y, Gao Y, et al. Emerging trends in organ-on-a-chip systems for drug screening. Acta Pharm Sin B. 2023. 13(6):2483-2509.
- Esch EW, Bahinski A, et al. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015. 14(4):248-60.
- Monteduro AG, Rizzato S, et al. Organs-on-chips technologies - A guide from disease models to opportunities for drug development. Biosens Bioelectron. 2023. 231:115271.

