Key Factors Influencing Brain Distribution of Drugs
Understanding how drugs distribute in the CNS is important to designing CNS-targeted therapies. Critical to the impact are the source of the drug (chemical or biological) and the uniqueness of the CNS, its anatomy, vasculature and fluid flow. This specialized environment of the CNS results in unique pharmacokinetics and pharmacodynamics, often deviating considerably from other body compartments. Neuropharmacokinetics (NPK) gives us a more accurate description of these dynamics, looking at how drugs are absorbed, distributed and eliminate within the CNS. Unlike general pharmacokinetic models that render the brain as an essentially monolithic structure sealed by the blood-brain barrier (BBB), NPK recognizes the CNS's multi-compartmental character: its brain, spinal cord, blood vessels and tissues.
Advancing CNS drug development necessitates integrating detailed pharmacokinetic profiles that consider the anatomical and physiological processes influencing drug delivery and action within the CNS. This comprehensive approach not only enhances the effectiveness of CNS-specific medications but also helps anticipate CNS-related side effects in non-targeted drugs, and some of the key factors are described below.
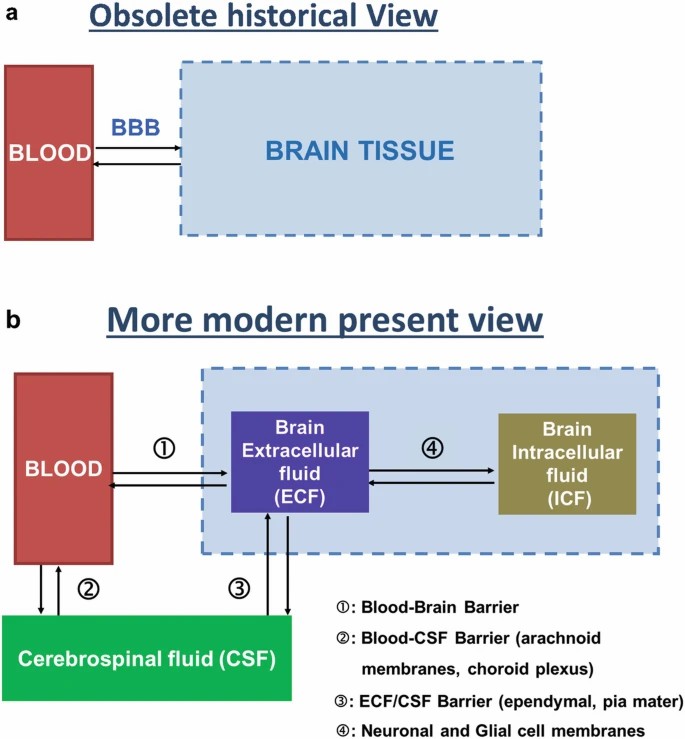 Fig. 1. Obsolete historical (a) and more modern present (b) views of the blood-brain compartments (Bourasset F, Auvity S, et al., 2022).
Fig. 1. Obsolete historical (a) and more modern present (b) views of the blood-brain compartments (Bourasset F, Auvity S, et al., 2022).
Brain Heterogeneity
Brain heterogeneity is a defining characteristic observed across different species, crucial to the development of central nervous system (CNS) therapies.
- Anatomical differences and regional heterogeneity
The human brain is anatomically divided into three main regions: the forebrain, midbrain, and hindbrain, each containing specific sub-regions or structures. These various areas have distinct functions, and brain disorders or diseases are typically associated with dysfunctions in one or several interconnected structures. The anatomical heterogeneity of the brain also depends on its circulating fluid components, including the blood-brain barrier (BBB) and additional fluid compartments within the brain, which may play a significant role in the heterogeneity of drug distribution.
- Cellular diversity in the brain
Astrocytes, microglia, oligodendrocytes and Schwann cells have been described as 2-10 times more abundant than neurons in the vertebrate forebrain. But more recent studies suggest that the ratio of neuron to glia differs between species and between CNS areas. Glial cells were once thought to provide supplementary functions for neurons, but now are understood to regulate blood flow, metabolism, homeostasis and CNS immunity. Other cells also play a key role in other CNS defences, including BBB endothelial cells and pericytes and epithelial cells in the choroid plexus, ependyma and arachnoid. In many therapeutic drugs, molecular targets are expressed only in neurons, but other cells, especially microglia, are also increasingly important targets for treating neurodegenerative conditions.
- Biochemical heterogeneity
In general, the brain's transport of many psychoactive chemicals is influenced by their separation into aqueous and lipid phases. Lipids represent about half of the brain's dry weight and are denser than all the other tissues in the body except fat tissue. Liposome content can also vary within a brain area – myelin, for instance, contains far more lipids than white or gray matter.
- Transporter heterogeneity
Drug transporters are proteins belonging to two superfamilies, the ATP-binding cassette (ABC) and solute carrier (SLC) transporter groups. These two superfamilies are made up of more than 400 proteins, some of which transport drugs. These proteins can transport their substrates either by facilitated diffusion (SLC) in the direction of the concentration gradient or by active transport against the concentration gradient. The brain expression of these proteins is ubiquitous, with certain quantitative and qualitative specificities. The heterogeneity of transporter expression at various brain interfaces can play a crucial role in the brain distribution of drugs.
Barriers in the CNS
Cerebral NPK depends on drug exchange between blood and brain fluids, i.e., blood, extracellular/intracellular brain fluids (ECF and ICF, respectively) and cerebrospinal fluid (CSF). These fluids communicate with each other via four brain interfaces: blood–BECF, blood–CSF, ECF-ICF and ECF/CSF.
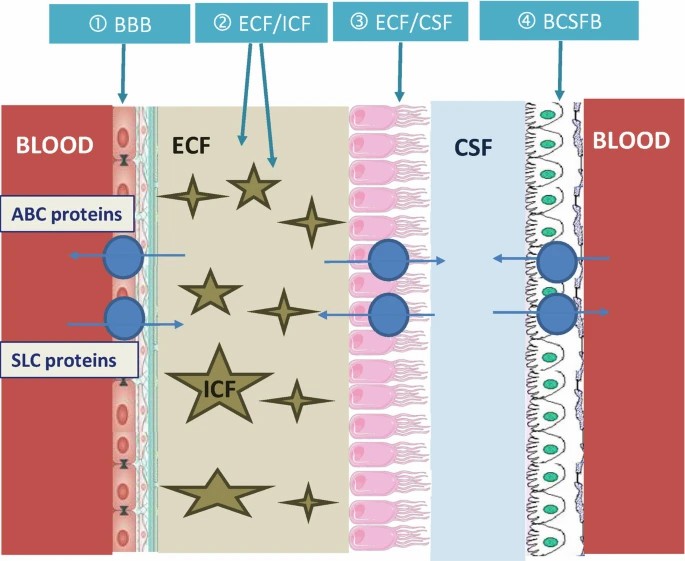 Fig. 2. Schematic representation of 4 key brain interfaces separating the blood and brain fluids (Bourasset F, Auvity S, et al., 2022).
Fig. 2. Schematic representation of 4 key brain interfaces separating the blood and brain fluids (Bourasset F, Auvity S, et al., 2022).
- Blood-brain barrier (BBB)
The blood-brain barrier is a key element in drug distribution in the brain. It is a selective permeability barrier that is made up of endothelial cells, pericytes and astrocytes. It helps control molecules' movement into the CNS by restricting access to bacteria and other toxic agents via its tight junctions (built from proteins such as claudin and occludin). These junctions provide an extremely high trans-endothelial electrical resistance that's essential for brain homeostasis. For passive diffusion, molecules need to be small, with hydrophilic and hydrophobic molecules requiring mass limits of 150 Da and 400-600 Da, respectively. Consequently, most molecules enter endothelial cells by endocytosis, facing destruction in lysosomes if they do not escape the endosomal pathway, a crucial step for effective CNS-targeted therapies.
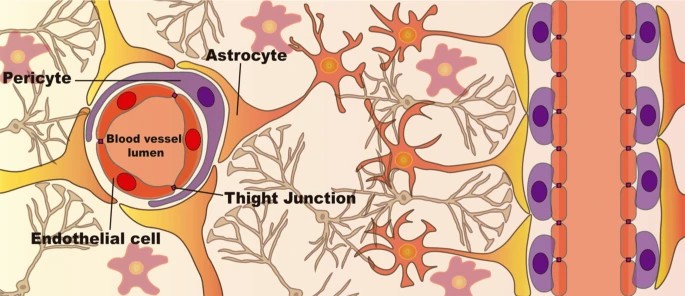 Fig. 3. Cellular structure of the Blood-Brain Barrier (BBB) (Moreira R, Nóbrega C, et al., 2024).
Fig. 3. Cellular structure of the Blood-Brain Barrier (BBB) (Moreira R, Nóbrega C, et al., 2024).
- The brain ECF-ICF barrier
The extracellular fluid (ECF), composed principally of plasma, inter-tissue fluid (also called intercellular fluid or interstitial fluid) and cerebrospinal fluid, is the medium that cells exist directly. The brain ECF-ICF barrier consists principally of neurons and glia's plasma membranes. These membranes are tagged with transporters and receptors, causing uneven dispersal of exogenous and/or endogenous molecules in ECF and ICF.
- The CSF-ECF barrier
It is composed primarily of the ventricular epithelium, limbic meninges and limbic meningeal subglia. This wall divides the cerebrospinal fluid from the extracellular fluid of the brain, and prevents some substances from flowing freely between the cerebrospinal fluid and the extracellular fluid of the brain.
- The Blood–CSF barrier
The barrier comprises choroid plexus epithelial cells and capillary endothelial cells. Of these, the capillary endothelial cells contain window pore structures, while the epithelial cells create a barrier of tight junctions that prevent some compounds from leaking into the cerebrospinal fluid.
| Products & Services | Description |
| Quantitative Tissue Distribution | Creative Bioarray provides quantitative tissue distribution services to help our customers visualize true tissue distribution, facilitate tissue PK analysis and dosimetry prediction before the initiation of human mass balance studies. |
| In Vivo Blood-Brain-Barrier Assay | Creative Bioarray has established a simple, reliable and efficient in vivo assay that has been successfully applied to several genetic and experimental mouse models. |
| Brain Tissue Binding Assay | Creative Bioarray provides Brain Tissue Binding Assay to help customers accurately detect the drug transport and distribution in the brain. |
References
- Moreira R, Nóbrega C, et al. Brain-targeted drug delivery - nanovesicles directed to specific brain cells by brain-targeting ligands. J Nanobiotechnology. 2024. 22(1):260.
- Bourasset F, Auvity S, et al. Brain Distribution of Drugs: Brain Morphology, Delivery Routes, and Species Differences. Handb Exp Pharmacol. 2022. 273:97-120.

