How to Improve the Pharmacokinetic Properties of Peptides?
Peptides, as therapeutic agents, offer an exciting frontier in modern pharmacotherapy due to their specificity and efficacy. But their widespread use is largely restricted by poor pharmacokinetics such as rapid degradability and shorter half-lives. This paper focuses on sophisticated methods to overcome these drawbacks, and improves the clinical utility of peptides.
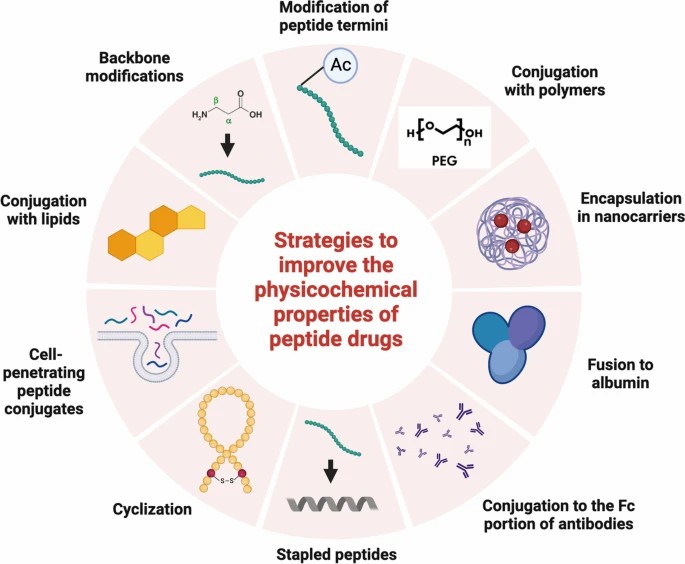 Fig. 1. Strategies to improve the physicochemical properties of peptide drugs (Lee MF and Poh CL, 2023).
Fig. 1. Strategies to improve the physicochemical properties of peptide drugs (Lee MF and Poh CL, 2023).
Strategies
Amino acid substitution
A mechanism used to improve the stability of peptides against enzymatic degradation is amino acid substitution. This strategy involves replacing the metabolically unstable natural L-amino acids with their mirror-image D-amino acids, N-alkylated amino acids, and various α- and β-substituted amino acids that resist proteolytic breakdown. Somatostatin, for example, which has a short half-life, has been adapted by replacing L-tryptophan with D-tryptophan, a synthetic equivalent with a far longer time-of-action. In a similar way, GnRH antagonists and agonists have combined synthetic amino acids to extend half-lives, including such medications as cetrorelix and triptorelin. While such strategies show promise, they also carry potential risks, including the possible induction of adverse effects or organ accumulation of unnatural amino acids, underscoring the importance of careful modifications.
Side chain modifications
Side chain modifications can improve peptides' binding ability and target selectivity by replacing natural amino acids with structurally equivalent ones during synthesis. Chemical modifications with analogs such as -phenylalanine, benzyloxytyrosine, and homoarginine are common.
Conjugation with polymers
Combining peptides with polymers is a viable strategy for improving stability and immunogenicity, and PEGylation is the most common approach due to its protective and longevity properties. PEGylation — a chemical process that binds peptides with polyethylene glycol (PEG) to shorten their circulatory duration and protect them from enzyme degradation (though there might be a safety issue with PEG, as it's non-biodegradable). In addition to PEG, biodegradable alternatives like polysialic acid (PSA) and XTEN offer non-toxic options without complicated chemical conjugation steps. While conjugation decreases efficacy, the prolonged exposure in the body can boost overall clinical efficacy through enhanced pharmacokinetics and biological activity.
Peptide termini modifications
Reversing peptide termini can dramatically improve peptide resistance to enzymatic oxidation, especially for peptides that are susceptible to exopeptidase. Techniques such as N-acetylation and C-amidation are typically utilized to achieve this stability.
Albumin fusion
Because of its large molecular weight (67 kDa), high concentration in blood plasma, and prolonged half-life, albumin is a natural candidate for fusion to prolong the half-life of therapeutic peptides. Peptide-albumin fusions such as CJC-1134 and albiglutide also highlight the ability to prolong the half-life of GLP-1 analogs.
Conjugation with antibody Fc portion
We can increase the survival of therapeutic peptides by attaching them to the Fc section of human antibodies and engaging the neonatal Fc receptor (FcRn) for protective recirculation. This method effectively extends their plasma lifetime by avoiding metabolic dissolution and elimination.
Stapled peptides
Stapled peptides are engineered into α-helix conformations through cross-linking, effectively mimicking protein interaction interfaces. As cell-penetrating molecules, they are antibody-like and immune to enzymatic degradation, prolonging plasma lifetime. These peptides can regulate intracellular protein interactions, which ALRN-5281 has shown can be used to treat some genetic diseases.
Cyclization
Cyclization enhances peptide stability by forming stable structures such as loops or turns, thus improving resistance to enzymatic degradation and increasing cell permeability. This process combines multiple segments of a peptide and stabilizes secondary structures such as α-helices and β-sheets.
Pseudopeptides
Peptide structure can be structurally altered by replacing the traditional peptide bond with electronic or structural counterparts for better stability. Azapeptides, retro-inverso peptides and peptoids are examples of such modifications. Azapeptides replace a nitrogen atom within the peptide structure, and are important for producing compounds such as protease inhibitors. Retro-inverso peptides reverse sequences and replace L-amino acids with D-amino acids for a range of therapeutic purposes, as well as potentially altering biological function. Peptoids manipulate nitrogen placement in the peptide chain, making them more malleable and helpful for applications such as enzyme inhibition and antimicrobial therapy.
Disulfide-rich peptides
Disulfide bridges ensure the structural integrity and folding of extracellular peptides and proteins. These bridges introduce conformational constraints that boost receptor selectivity and potency, as well as thermostability.
Conjugation with cell-penetrating peptides
Cell-penetrating peptides (CPPs) usually consist of less than 30 amino acids and they pass through biological membranes efficiently. They vary widely and can be broadly classified into the categories of cationic and amphiphilic: oligoarginine, penetratin, HIV-TAT1. High levels of basic amino acids, such as arginine and lysine, enable interaction with negatively charged glycosaminoglycans on cell surfaces, facilitating membrane penetration. CPP uptake is largely receptor-independent, through endocytosis or direct penetration. Conjugation of CPPs with cargo molecules, like insulin, can facilitate membrane crossing but sometimes interfere with CPP function.
Conjugation with lipids
Adding lipids to medications dramatically improves their properties by reducing toxicity and increasing oral bioavailability and efficacy. Lipid conjugation involves linking drugs to lipids, such as fatty acids, steroids, or triglycerides. One widely used method is splicing the carboxyl side of fatty acids, such as squalenoic or palmitic acid, into permanent bonds that increase drug stability and circulation time.
| Products & Services | Description |
| Quantitative Tissue Distribution | Creative Bioarray provides quantitative tissue distribution services to help our customers visualize true tissue distribution, facilitate tissue PK analysis and dosimetry prediction before the initiation of human mass balance studies. |
| In Vitro Metabolism | With extensive knowledge and experience in drug discovery, Creative Bioarray can provide standard, cost-effective in vitro metabolism services, including drug metabolic stability services and drug-drug interaction services to support your drug development process. |
| Pharmacokinetic and Toxicokinetic Studies | Creative Bioarray provides professional PK/TK testing services to help our customers choose pharmaceutical compounds and effective and safe dosing regimens. |
References
- Diao L, Meibohm B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin Pharmacokinet. 2013. 52(10):855-68.
- Lee MF, Poh CL. Strategies to improve the physicochemical properties of peptide-based drugs. Pharm Res. 2023. 40(3):617-632.

