How to Design and Synthesize Antibody Drug Conjugates?
Over the past years, antibody-drug conjugates (ADCs) have rapidly become important in the oncology therapeutic space. They represent a significant advancement, merging the specificity of monoclonal antibodies with the potency of cytotoxic drugs. These innovative therapeutic agents are designed to selectively deliver chemotherapeutic agents to cancer cells while minimizing systemic toxicity.
Approved ADCs by 2024
As of July 2024, 15 ADC drugs have been approved by regulatory authorities worldwide, covering multiple indications such as hematologic tumors and solid tumors.
| ADC drug | Payload/payload class | Payload action | Target | mAb | Linker | DAR |
| Mirvetuximab soravtansine | Maytansinoid DM4 | Microtubule inhibition | FRα | lgG1 | Enzyme cleavable | 3.5 |
| Tisotumab vedotin-tftv | MMAE/auristatin | Microtubule inhibition | Tissue factor | lgG1 | Enzyme cleavable | 4 |
| Disitamab vedotin | MMAE/auristatin | Microtubule inhibition | HER2 | lgG1 | Enzyme cleavable | 4 |
| Cetuximab sarotalocan | Photosensitizing dye | Damage cell membrane | EGFR | Chimeric lgG1 | Non-cleavable | 1.3-3.8 |
| Loncastuximab tesirine-lpyl | SG3199/PBD dimer | DNA damage | CD19 | lgG1 | Enzyme cleavable | SG3199/PBD dimer |
| Belantamab mafodotin-bimf | MMAF/auristatin | Microtubule inhibition | BCMA | lgG1 | Non-cleavable | 4 |
| Sacituzumab govitecan | SN-38/camptothecin | TOP1 inhibition | TROP2 | lgG1 | Acid cleavable | 7.6 |
| Trastuzumab deruxtecan | Dxd/camptothecin | TOP1 inhibition | HER2 | lgG1 | Enzyme cleavable | 8 |
| Enfortumab vedotin | MMAE/auristatin | Microtubule inhibition | Nectin 4 | lgG1 | Enzyme cleavable | 3.8 |
| Polatuzumab vedotin-piiq | MMAE/auristatin | Microtubule inhibition | CD79 | lgG1 | Enzyme cleavable | 3.5 |
| Moxetumomab pasudotox | PE38 (Pseusotox) | Immunotoxin | CD22 | lgG1 | Cleavable | N/A |
| Inotuzumab ozogamicin | Ozogamicin/calicheamicin | DNA damage | CD22 | lgG4 | Acid cleavable | 6 |
| Trastuzumab emtansine | DM1/maytansinoid | Microtubule inhibition | HER2 | lgG1 | Non-cleavable | 3.5 |
| Brentuximab vedotin | MMAE/auristatin | Microtubule inhibition | CD30 | lgG1 | Enzyme cleavable | 4 |
| Gemtuzumab ozogamicin | Ozogamicin/calicheamicin | DNA damage | CD33 | lgG4 | Acid cleavable | 2-3 |
Components of ADCs
ADCs comprise three main components: monoclonal antibodies (mAbs), cytotoxic drugs, and linkers.
 Fig. 1 Schematic representation of an ADC. (Yao H, et al., 2016)
Fig. 1 Schematic representation of an ADC. (Yao H, et al., 2016)
Monoclonal antibodies (mAbs)
mAbs serve as the targeting arm of ADCs, recognizing and binding to specific antigens expressed on the surface of tumor cells. The choice of mAb is crucial, as it determines the ADC's specificity and potential to elicit an immune response. For instance, trastuzumab is an ADC targeting HER2-positive breast cancer, demonstrating significant clinical success.
Cytotoxic drugs
The cytotoxic component is typically a small-molecule chemotherapy agent that disrupts cancer cell function. Commonly used drugs include auristatins and maytansinoids, which are known for their potent anti-proliferative effects. The selection of the cytotoxic drug is guided by its mechanism of action and ability to induce cell death upon internalization.
Linkers
Linkers are pivotal in ADC design, acting as the bridge between the antibody and the drug. They can be classified as cleavable or non-cleavable. Cleavable linkers are designed to release the drug inside the target cell, while non-cleavable linkers remain intact until the ADC is degraded. The stability and release characteristics of the linker significantly influence the ADC's therapeutic index.
ADC Design Considerations
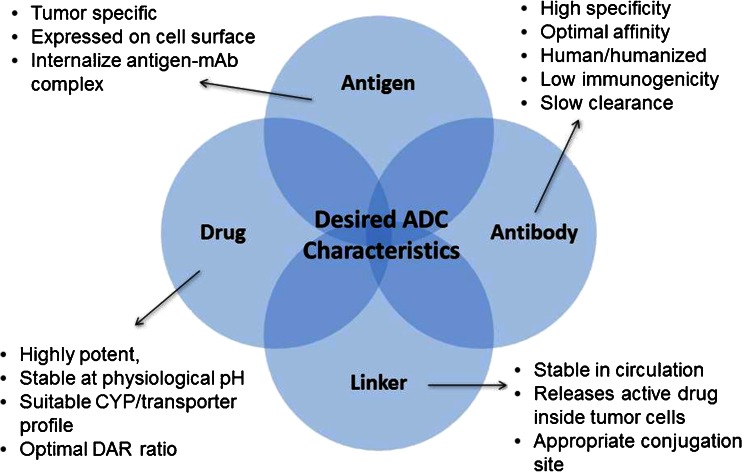 Fig. 2 Desired attributes of the components of an ADC. (Kamath AV and Iyer S, 2015)
Fig. 2 Desired attributes of the components of an ADC. (Kamath AV and Iyer S, 2015)
Target selection
The selection of the target antigen is foundational in ADC design. Ideal targets are overexpressed in tumor cells but have limited expression in normal tissues. This specificity helps to enhance the ADC's safety profile. For example, CD30 is a target for ADCs in Hodgkin lymphoma, where its expression is primarily restricted to malignant cells.
Linker design
Linker design involves careful consideration of several factors, including stability in circulation, release kinetics, and solubility. The linker must remain stable in the bloodstream to prevent premature drug release but should cleave efficiently once internalized by the cancer cell. The choice between cleavable and non-cleavable linkers often depends on the intended therapeutic strategy.
Drug-to-antibody ratio (DAR)
The drug-to-antibody ratio (DAR) is a critical parameter impacting ADC efficacy and safety. A higher DAR can enhance cytotoxicity but may also increase off-target effects. Balancing DAR is essential; a well-optimized ADC aims for a DAR that maximizes therapeutic benefit while minimizing toxicity.
ADC Synthesis Methods
Non-specific conjugation through native residues
Reactive side chains of naturally occurring amino acids such as lysine and cysteine are attractive sites of conjugation. The main advantage of linkage through native residues is facile reactivity that does not require preliminary processing/modification of the antibody. The main disadvantages of these methods are the variability and heterogeneity of the resulting products.
Site-specific conjugation through genetically engineered sites
To increase the site specificity of ADC conjugation, reactive handles can be introduced by altering amino acid sequences. Several limitations of this method may be avoided by incorporating more discriminate residues, especially unnatural amino acids. Alternatively, ligating enzymes can be used to catalyze bond formation between specific sequences or chemical groups.
| Methods | Descriptions |
| Conjugation via thiols | One prevalent method for ADC synthesis involves thiol-based conjugation, which exploits the reactivity of free cysteine residues in mAbs. This technique allows for selective drug attachment to the antibody, ensuring that the functional integrity of the mAb is maintained. |
| Conjugation via amines | Amine conjugation is another widely used approach, where the drug is linked to lysine residues on the antibody. This method is advantageous due to the abundance of amine groups, enabling multiple drug attachments. However, it can lead to heterogeneous products, necessitating careful characterization. |
| Conjugation via alcohols | Conjugation through alcohols involves the use of serine or threonine residues, which can be selectively modified. This method can result in stable ADCs with defined structures, which are crucial for consistent therapeutic performance. |
| Conjugation via aldehydes | Aldehyde conjugation is a less common but effective technique, where sugars on the antibody surface are oxidized to form aldehyde groups. This allows for the incorporation of drugs via oxime or hydrazone linkages, providing stability and controlled release. |
| Conjugation via azides | The use of azide groups in click chemistry has opened new avenues for ADC synthesis. This method allows for precise and efficient conjugation, enabling the formation of stable linkages while minimizing by-products. The versatility of click chemistry is advantageous for creating ADCs with tailored properties. |
Techniques for Characterizing ADCs
UV/Vis spectroscopy
UV/Vis spectroscopy is a fundamental technique for assessing the purity and concentration of ADCs. By measuring absorbance at specific wavelengths, researchers can quantify the antibody and drug components, providing insight into the overall quality of the ADC.
Chromatography
Chromatographic techniques, such as high-performance liquid chromatography (HPLC), are essential for separating and analyzing ADCs. HPLC can identify different species within the ADC preparation, including unconjugated antibodies and drug aggregates, ensuring that only the desired product is used in therapeutic applications.
Mass spectrometry
Mass spectrometry offers a powerful tool for characterizing the molecular weight and structure of ADCs. This technique provides detailed information on the DAR, linker stability, and the presence of modifications, which are crucial for understanding the ADC's pharmacokinetics and dynamics.
Creative Bioarray Relevant Recommendations
| Service Types | Description |
| In Vitro DMPK Services | Creative Bioarray provides a variety of in vitro ADME/PK services, including high-throughput ADME screening, in vitro binding, in vitro metabolism, in vitro permeability, and transporter assays. |
| In Vivo DMPK Services | Creative Bioarray's scientific team provides expertise in DMPK studies at all stages of R&D. We have extensive experience in designing, performing, and interpreting the results of DMPK studies in multiple species. |
References
- Yao H, et al. (2016). "Methods to Design and Synthesize Antibody-Drug Conjugates (ADCs)." Int J Mol Sci. 17 (2): 194.
- Kamath AV, Iyer S. (2015). Preclinical Pharmacokinetic Considerations for the Development of Antibody Drug Conjugates. Pharm Res. 32 (11): 3470-9.

