Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Oral Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
Oral tumor cells are a crucial aspect of research in head and neck oncology, reflecting a variety of pathological changes in the tissues of the oral cavity. These cells primarily arise from the squamous epithelium, making oral squamous cell carcinoma (OSCC) the most common malignant tumor in this region. The exploration of oral tumor cells not only underscores the biological mechanisms underpinning their development but also paves the way for innovative therapeutic strategies and diagnostic techniques.
Biological Characteristics of Oral Tumor Cells
- Dysregulated cell cycle. Tumor cells often exhibit uncontrolled proliferation due to mutations in oncogenes and tumor suppressor genes. For instance, alterations in genes like TP53 disrupt cell cycle regulation, leading to increased cellular division and tumor growth.
- Invasive behavior. Many oral tumor cells acquire the ability to invade surrounding tissues, a hallmark of malignancy. This invasive potential is facilitated by changes in cell adhesion molecules and the extracellular matrix.
- Metabolic changes. Cancer cells often undergo metabolic reprogramming, favoring glycolysis over oxidative phosphorylation, a phenomenon known as the Warburg effect. This shift supports rapid growth and survival in a hypoxic environment.
Diagnostic and Prognostic Markers
Oral tumor cells serve as critical tools in the development of diagnostic and prognostic markers. Researchers have identified specific molecular signatures and genetic alterations in oral tumor cells that can predict disease progression and response to treatment. These markers are not only valuable for clinical decision-making but also for the design of personalized therapeutic approaches.
Therapeutic Targets
The investigation of oral tumor cells has led to the identification of novel therapeutic targets. Research has been instrumental in characterizing key signaling pathways and oncogenes that are pivotal to the survival and growth of oral tumor cells. By targeting these pathways, scientists aim to develop new treatments that are more effective and less toxic than traditional modalities.
Immunotherapy and Vaccines
The field of immunotherapy has seen significant advancements, with oral tumor cells playing a central role in the development of novel immunotherapies and vaccines. Researchers are exploring the use of tumor-infiltrating lymphocytes and cancer vaccines to stimulate the immune system to recognize and attack oral tumor cells. These approaches hold the promise of a more durable and less invasive form of treatment for patients with oral cancer.
MiR-205 Induces Cell Apoptosis Through the Activation of Caspase-3/-7 in Human KB Oral Cancer Cells
Miscellaneous studies have examined the biological functions of microRNA-205 (miR-205) as a tumor suppressor in carcinogenesis. the pSuper-miR-205 construct was generated to over-express the miR-205 and transfected into the cultured KB cells. The level of cell viability at 200 ng/ml pSuper-miR-205 was monitored for 72 h. As shown in Fig. 1A (Upper panel), the transfected KB cells with 200 ng/ml pSuper-miR-205 showed approximately lower cell viability by 40% (p < 0.05) compared to the untransfected and pSuper-miR-205 mutation. The up-regulated miR-205 did not alter the cell viability in the NHOKs. Therefore, the viability of the oral KB oral cancer cells was monitored for 72 h after transfection with pSuper-miR-205 according to the dose. As shown in Fig. 1A (Lower panel), up-regulated miR-205 decreased the cell viability in a dose-dependent manner. The KB cells transfected with 200 ng/ml of pSuper-miR-205 exhibited 40% lower cell viability (p < 0.05), as shown in the upper panel data in Fig. 1A. The cell death induced by the up-regulated miR-205 in KB cells was examined further by DAPI staining, which detects the nuclear morphology. As shown in Fig. 1B, the number of cells with the typical morphology of cell death, such as condensed and/or fragmented nuclei, increased under the over-expressed miR-205. Cell death was not detected in either the untransfected or mock cells. The percentage of cell death is presented as a histogram. As shown in Fig. 1B (lower panel), the level of cell death was at least 10-fold higher than either the untransfected or pSupermiR-205 mutation.
To determine if miR-205 has proapoptotic properties, the level of caspase-3/-7 activation was examined as a possible cell death mechanism in KB cells with up-regulated miR-205. As shown in Fig. 1C, the KB cells containing up-regulated miR-205 showed a marked increase in the number of caspase-3/-7-positive apoptotic cells. Moreover, the typical morphology of KB cells was lost and the number of KB cells was decreased significantly by the up-regulated miR-205 compared to the untransfected and pSuper-miR-205mutation. In contrast, neither untransfected nor pSuper-miR-205 mutation activated caspase-3/-7 or altered the cell morphology compared to the KB cells with up-regulated miR-205. Therefore, up-regulated miR-205 induced oral cancer KB cell death through the activation of caspase-3/-7. An immunoblotting assay for the expression of the apoptotic factors, such as PARP and cleaved caspase-3, was performed to confirm the miR-205-induced apoptosis in KB cells. Over-expressed miR-205 significantly cleaved PARP more than the pSuper-miR-205 mutation. In addition, in the pSuper-miR-205 transfected cells, cleaved caspase-3 was expressed significantly more than the pSuper-miR-205 mutation. Therefore, overexpressed miR-205 induces the apoptosis of KB oral cancer cells.
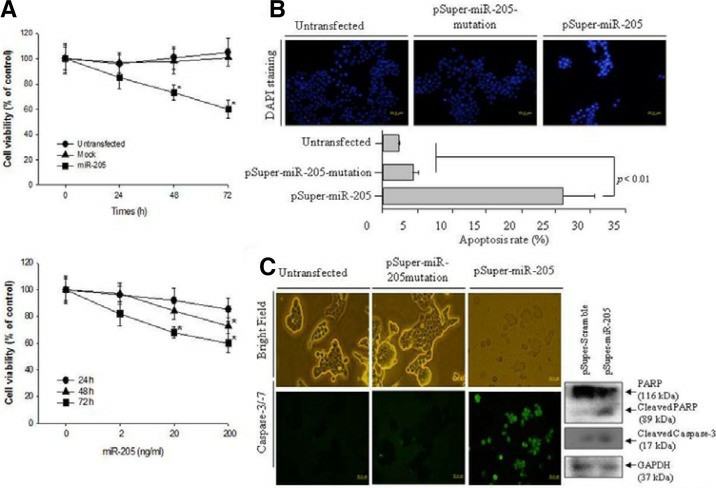 Fig. 1 Over-expressed miR-205 increased the cell cytotoxicity via apoptotic cell death in KB oral cancer cells. (A) The measurement of cell cytotoxicity by up-regulated miR-205 as time-dependent (upper panel) and dose-dependent manner (low panel). (B) The observation of apoptotic cell death was performed by DAPI staining after transfection of 200 ng/ml pSuper-miR-205 and 200 ng/ml pSuper-miR-205mutation. (C) Apoptosis executioner caspase-3/7 activity was determined using the cell-permeable fluorogenic substrate. (Kim JS, et al, 2012)
Fig. 1 Over-expressed miR-205 increased the cell cytotoxicity via apoptotic cell death in KB oral cancer cells. (A) The measurement of cell cytotoxicity by up-regulated miR-205 as time-dependent (upper panel) and dose-dependent manner (low panel). (B) The observation of apoptotic cell death was performed by DAPI staining after transfection of 200 ng/ml pSuper-miR-205 and 200 ng/ml pSuper-miR-205mutation. (C) Apoptosis executioner caspase-3/7 activity was determined using the cell-permeable fluorogenic substrate. (Kim JS, et al, 2012)
Changes in Gene Expression in Co-culture of Normal PDL Fibroblasts With SCC-25 Cells
Fibroblast (Fibs) contribution to neoplastic progression, tumor growth, angiogenesis, and metastasis has been recently reported. In the co-culture, fibroblasts were converted to carcinoma-associated fibroblasts (CAFs), which in return initiated epithelial-mesenchymal transition (EMT) of SCC-25 lingual squamous cell carcinoma cells. Normal human periodontal filament fibroblasts (PDLs), and SCC-25 cells were co-cultured for 7 days. After this time gene expression changes in PDLs and SCC-25 cells were investigated, and compared with control cells. The controls received the same cultural conditions, but they were not co-cultured with the partners. By this analysis, in the case of fibroblasts, changes in stroma-derived factor-1 (SDF-1) were investigated in co-culture vs. controls. 1.6-times significant (p < 0.01 by one-way ANOVA) reproducible increase of SDF expression was found in co-cultured fibroblasts compared with control PDL fibroblasts, which indicates the development of carcinoma-associated fibroblasts of PDLs. In the same co-culture system gene expression changes of key genes for EMT were investigated in co-cultured SCC-25 cells vs. control SCC-25 cells. A significant increase in vimentin mRNA expression was detected (Fig. 2A), while E-cadherin mRNA expression was dramatically decreased (Fig. 2B) (p < 0.05). The snail was detected in the control and co-cultured SCC-25 cells; its gene expression increase in co-culture was not statistically significant (p = 0.27) (Fig. 2C). Significant (p < 0.01) correlation was found between gene expression of vimentin and snail (correlation coefficient: 0.95). A significant (p < 0.01) negative correlation was found between the gene expression of vimentin and E-cadherin (correlation coefficient: -0.81), and a statistically significant (p = 0.05) correlation was found between the gene expression of snail and E-cadherin (correlation coefficient: 0.37). These changes represent the characteristic gene expression changes involved in EMT.
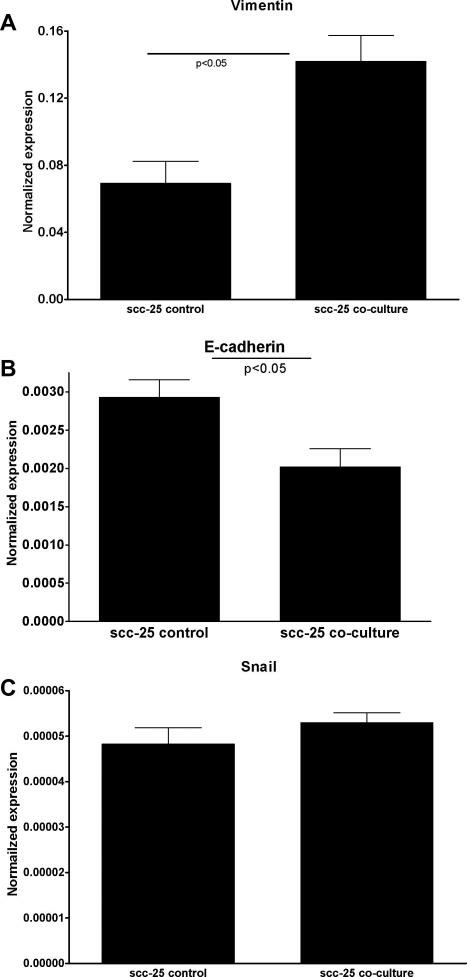 Fig. 2 EMT-related gene expression changes in co-cultured SCC-25 cells. Key genes for EMT were investigated in co-cultured SCC-25 cells vs. control SCC-25 cells. A significant increase in vimentin mRNA expression was detected (A), while E-cadherin mRNA expression was dramatically decreased (B). The snail was detected in the control and co-cultured SCC-25 cells; its gene expression increase in co-culture was not statistically significant (C). (Dudás J, et al., 2010)
Fig. 2 EMT-related gene expression changes in co-cultured SCC-25 cells. Key genes for EMT were investigated in co-cultured SCC-25 cells vs. control SCC-25 cells. A significant increase in vimentin mRNA expression was detected (A), while E-cadherin mRNA expression was dramatically decreased (B). The snail was detected in the control and co-cultured SCC-25 cells; its gene expression increase in co-culture was not statistically significant (C). (Dudás J, et al., 2010)
Description: Squamous carcinoma cells derived from human oral cancer.
Description: Human oral squamous carcinoma established from xenotransplantation in mice.
Description: Human tumor cells derived from oral cancer at stage III. The cells were originally...
Description: The ES-2 cell line was established from a surgical tumor specimen taken from a 47...
Description: Growth of SCC-9 is enhanced by using a feeder layer of Mitomycin C-treated STO cells
Description: The FaDu cell line which is from a squamous cell carcinoma of the hypopharynx and...

