Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Brain/Nerve Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
- FAQ
The human brain, the command center of the nervous system, is a complex organ composed of various types of cells, including nerve cells (neurons) and supporting cells (neuroglia). Brain and nerve tumor cells are a unique class of cancer cells that originate from the various cell types found in the central and peripheral nervous systems. These include glioma cells, which are derived from glial cells, and neuroblastoma cells, which arise from neural crest cells. These tumor cells exhibit distinct morphological, genetic, and behavioral characteristics that set them apart from other types of cancer cells.
Types of Brain/Nerve Tumor Cells
- Glioma cells. They are the most common type of brain tumor cells, originating from glial cells in the central nervous system. These include astrocytoma cells, oligodendroglioma cells, and ependymoma cells, among others. Glioma cells exhibit a wide range of malignancy grades, from low-grade (grade I-II) to highly aggressive, high-grade (grade III-IV) glioblastoma multiforme (GBM) cells.
- Neuroblastoma cells. They are derived from neural crest cells and are the most common extracranial solid tumors in children. These cells are found in the sympathetic nervous system and are known for their aggressive and heterogeneous nature. Neuroblastoma cells can exhibit varying degrees of differentiation, from highly undifferentiated and aggressive forms to more differentiated and less aggressive subtypes.
- Meningioma cells. They originate from the meningeal cells that line the outer covering of the brain and spinal cord. While often considered benign, some meningioma subtypes can exhibit more aggressive behavior and are associated with a higher risk of recurrence.
- Schwannoma cells. They are derived from Schwann cells, which are responsible for the myelination of peripheral nerves. These tumor cells are commonly associated with conditions like neurofibromatosis and can lead to the development of benign or malignant peripheral nerve sheath tumors.
- Pituitary adenoma cells. They originate from the anterior pituitary gland and can lead to the development of pituitary adenomas, which are often benign but can still cause significant hormonal imbalances and neurological complications.
Drug discovery and screening
One of the primary applications of brain and nerve tumor cells is in the field of drug discovery and screening. These specialized cells provide an excellent in vitro model for testing the efficacy and safety of potential therapeutic compounds targeting brain and nervous system cancers. Exposing brain and nerve tumor cells to various drugs or drug candidates, researchers can gain crucial insights into their mechanisms of action, identify potential targets, and evaluate the susceptibility of different tumor subtypes to specific treatments.
Genetic and molecular profiling
Brain and nerve tumor cells are also extensively used in genetic and molecular profiling studies. Analyzing the unique genomic, epigenomic, and transcriptomic profiles of these cells, researchers can uncover the underlying genetic alterations and signaling pathways that drive the development and progression of brain and nervous system cancers. This knowledge is essential for the development of personalized treatment strategies and the identification of novel therapeutic targets.
Tumor heterogeneity and microenvironment
Another important aspect of brain and nerve tumor cell research is the exploration of tumor heterogeneity and the tumor microenvironment. These specialized cells often exhibit significant intra- and inter-tumoral heterogeneity, reflecting the complex and dynamic nature of brain and nervous system cancers. By studying the interactions between brain and nerve tumor cells and their surrounding stromal components, such as immune cells and the extracellular matrix, researchers can gain a deeper understanding of the factors that influence tumor growth, invasion, and response to treatment.
Preferential Glucose Uptake by BTICs Under Restricted Nutrition
Like all cancers, brain tumors require a continuous source of energy and molecular resources for new cell production. De novo synthesis of molecular building blocks for cell growth requires glucose for energy production. Highly efficient glucose uptake could therefore provide a competitive advantage contributing to tumor maintenance, particularly with restricted glucose levels.
A glucose oxidase colorimetric assay after a pulse post nutrient starvation to measure glucose in cell lysates demonstrated brain tumor-initiating cells (BTICs) had higher levels of glucose than matched non-BTICs (Fig. 1a). To directly compare the uptake of glucose in GBM tumor cell subsets, a fluorescent glucose analog, 2-[N-(7-nitrobenz-2-oxa-1,3-diaxol-4-yl)amino]-2-deoxyglucose (2-NBDG) that mimics glucose19 was used. Bulk GBM cells were incubated with 2-NBDG and an antibody against the BTIC marker CD133. Flow cytometry demonstrated that CD133 expressing cells consistently displayed higher 2-NBDG fluorescence (Fig. 1b). Ex vivo multiphoton imaging of labeled GBM subpopulations in brain slice cultures confirmed that BTICs outcompete for glucose with surrounding brain and differentiated tumor cells (Fig. 1c-g). Quantification of fluorescence visualized through multiphoton imaging with Imaris reconstruction20 demonstrated that the uptake of NBDG was greater than two-fold higher in the BTICs, consistent with results in the flow cytometry assays. Together, these data indicate that BTICs preferentially uptake glucose relative to their non-BTIC counterparts.
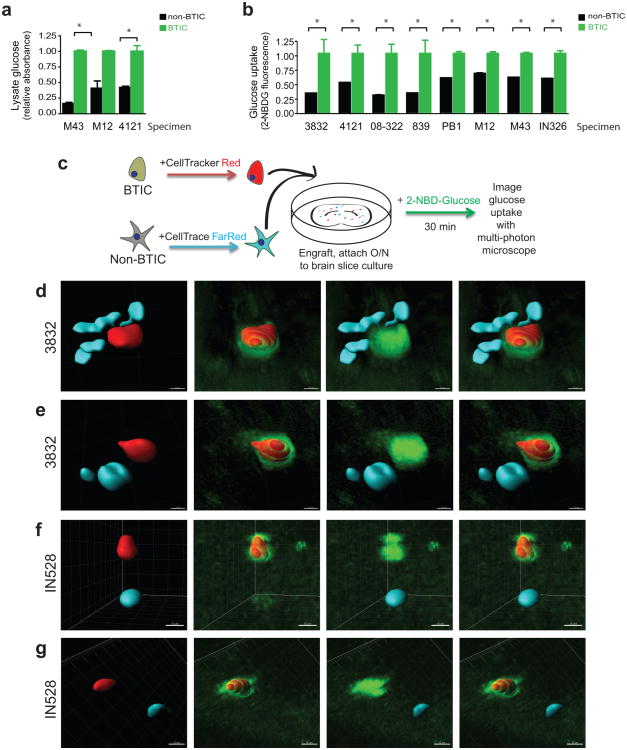 Fig. 1 BTICs preferentially uptake glucose. (Flavahan WA, et al, 2013)
Fig. 1 BTICs preferentially uptake glucose. (Flavahan WA, et al, 2013)
PRMT5 Inhibition Induces Aberrant Alternative Splicing in Glioblastoma
Glioblastoma (GBM) is a deadly cancer in which cancer stem cells (CSCs) sustain tumor growth and contribute to therapeutic resistance. Protein arginine methyltransferase 5 (PRMT5) has recently emerged as a promising target in GBM.
To better understand the cellular mechanisms that may account for the attenuation of growth observed upon PRMT5 inhibition in GSCs, bulk RNA-seq was performed on three GSC lines (G561, G564, and G583) after a 3-day treatment with GSK591 or the inactive control, SGC2096, and analyzed the differential effect on gene expression. 646 genes were identified that were significantly differentially expressed between the two treatments (false discovery rate (FDR)<0.05) (Fig. 2a). Gene set enrichment analysis (GSEA) of the differentially expressed genes (DEGs) revealed enrichment in genes involved in spliceosome complex-related pathways, corroborating previous reports that PRMT5 inhibition has a profound effect on the maintenance of splicing fidelity through disruption of arginine methylation of splicing factors and RNA-binding proteins.
RNA-seq data from the above three GSC lines were analyzed to identify disruptive alternative splicing events (ASEs). A total of 11,582 statistically significant differentially spliced events were identified in the three GSC lines upon PRMT5 inhibition. Only a fraction of these events (3%, 317 events) were common in all three GSC lines, suggesting that, although PRMT5 inhibition leads to widespread splicing disruption in the GSC lines, the alternatively spliced transcripts varied widely from sample to sample (Fig. 2c). The 317 common ASEs (occurring in 274 genes) included cassette exons (CEs), mutually exclusive exons (MXEs), alternative splicing at the 3' or 5' site (A3/5SSs), and retained introns (RIs), with the highest number of ASEs comprising CEs and Ris. Analysis of the predicted protein impact of the ASEs by VASTdb revealed that 69% of ASEs had predicted disruptive effects on the proteins they encode, with CEs and RIs resulting in the most deleterious impact on their targets (Fig. 2d). Relative to the null expectation whereby each ASE should be equally likely to be included or excluded after treatment, independently of its predicted effect, ASEs observed after PRMT5 inhibition were significantly more often predicted to cause open reading frame (ORF) disruption.
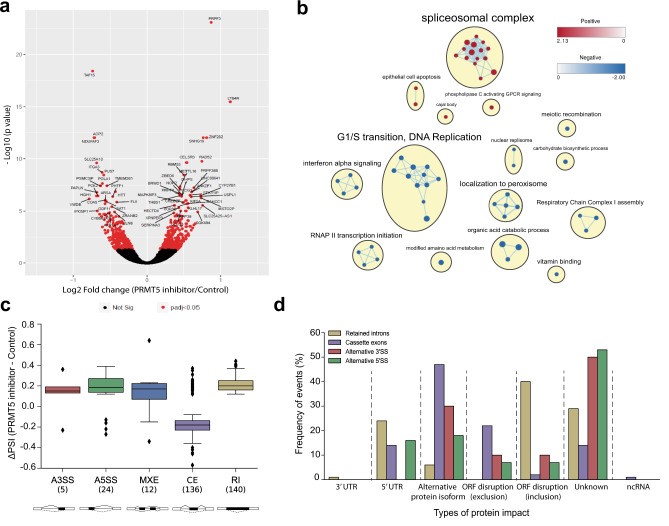 Fig. 2 PRMT5 inhibition leads to the deregulation of alternative splicing. (Sachamitr P, et al., 2021)
Fig. 2 PRMT5 inhibition leads to the deregulation of alternative splicing. (Sachamitr P, et al., 2021)
Brain and nerve tumor cells exhibit genetic and molecular alterations that lead to uncontrolled cell growth, increased proliferation, and invasion of surrounding tissues. They often have impaired mechanisms for regulating cell division and survival, setting them apart from their healthy counterparts.
Well-characterized brain and nerve tumor cell lines, such as those provided by Creative Bioarray, offer several advantages for researchers, including the availability of a consistent and reliable source of cells, the ability to conduct reproducible experiments, and the opportunity to study specific subtypes of brain and nerve tumors in a controlled and standardized manner.
Studying brain and nerve tumor cells can be challenging due to their inherent genetic and cellular heterogeneity, as well as the complex tumor microenvironment that influences their behavior. Additionally, the location and accessibility of brain and nervous system tumors can pose practical difficulties for researchers.
Description: Established 1974 from lung metastases obtained at post mortem from a 2 1/2-year-old boy with neuroblastoma
Description: Established from the adrenal tumor tissue resected after treatment from a 20-month-old...
Description: Established in 2011 from a metastasized group 3 medulloblastoma in the cerebellum...
Description: Human cell line derived from malignant peripheral nerve sheath tumor (MPNST).
Description: Human cell line derived from Hodgkin's lymphoma.
Description: Human cell line derived from neuroblastoma. Dibuty-cAMP (1 mM) induces differentiation.
Description: Human cell line derived from astrocytoma developed in a patient with NF-1 (neurofibromatosis type 1)
Description: Human brain derived lymphoma. Epstein-Barr virus positive.
Description: Subline of GOTO. Protein-free medium adapted. Cell growth is slow.
Description: Subline of U-251 MG with epithelial morphology.
Description: Human cell line with primitive neuroectodermal tumor (Askin's tumor).

