Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
LC-2/ad
Cat.No.: CSC-C6512J
Species: Human
Morphology: epithelial-like
Culture Properties: Adherent cells
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Store in liquid nitrogen.
A new human cell line, LC-2/ad was established from the pleural effusion of pulmonary adenocarcinoma of a 51-year-old Japanese female. The LC-2/ad cells exhibit an epithelial appearance and a tendency to form small domes as observed with phase-contrast microscopy. The modal chromosome number was 53-56. Plating efficiency and doubling time were 6.8% and 58 h, respectively (32th passage). Immunocytochemically, the cells were strongly positive for CEA and cytokeratins including cytokeratin no. which is present in simple epithelia. Ultrastructurally, the cultured cells were characterized by well-formed junctional complexes and microvilli. Subcutaneous injection of 5x106 cells into a nude mouse resulted in tumor formation classified histologically as a moderately differentiated adenocarcinoma. This cell line produced at least two functionally active trypsin inhibitors together with several proteinases in vitro. The main inhibitor was purified partially from the serum-free conditioned medium and confirmed immunologically as human alpha 1-antitrypsin (AAT). Immunohistochemically, the xenografted tumor was also positive for AAT. The cell line LC-2/ad is useful for the study of tumor-derived serine proteinase inhibitors, in particular AAT.
Identification of CCDC6-RET Fusion in the LC-2/ad Cell Line
KRAS mutations, epidermal growth factor receptor (EGFR) mutations, and anaplastic lymphoma receptor tyrosine kinase (ALK) fusions are well-known driver mutations identified in individuals with lung adenocarcinoma. Recently, several independent studies newly identified RET fusions (KIF5B-RET or CCDC6-RET) in approximately 1% of patients with primary lung tumors. RET fusions were mutually exclusive of EGFR, KRAS, and ALK mutations. First, in our oligonucleotide array analysis data of 39 cell lines, LC/2-ad showed lower expressions of vascular endothelial growth factor receptor-2 and EGFR, but distinctively higher expression of RET than that of other cell lines (Fig. 1A). Second, the result of an independently performed exon array analysis of LC-2/ad suggested the existence of RET fusion in LC-2/ad.
To directly demonstrate RET fusion in LC-2/ad, fusion-specific RT-PCR was performed with primers sets that detect RET-CCDC6 or RET-KIF5B. RET-CCDC6 fusion, but not RET-KIF5B fusion, was detected in LC-2/ad. No PCR product was amplified in any of the other cell lines examined (Fig. 1B and C). Direct sequence analysis of RT-PCR products demonstrated a fusion between CCDC6 exon1 and RET exon12 (Fig. 1D). An independently performed 5' rapid amplification of complementary DNA ends (5'-RACE) using a primer complementary to RET demonstrated the same fusion in LC-2/ad. Although CCDC6-RET fusions have now been found in many lung cancers, these cases have the same breakpoints; exon 1 of CCDC6 is fused to exon 12 of RET at the cDNA level. The thyroid cancer cell line TPC-1 also harbors CCDC6-RET fusion at the same site. However, the breakpoints of the RET and CCDC6 genes were not checked in LC-2/ad at the genomic level. Thus, it would be of interest to further determine and characterize the genomic breakpoints in CCDC6-RET fusion-positive tumors.
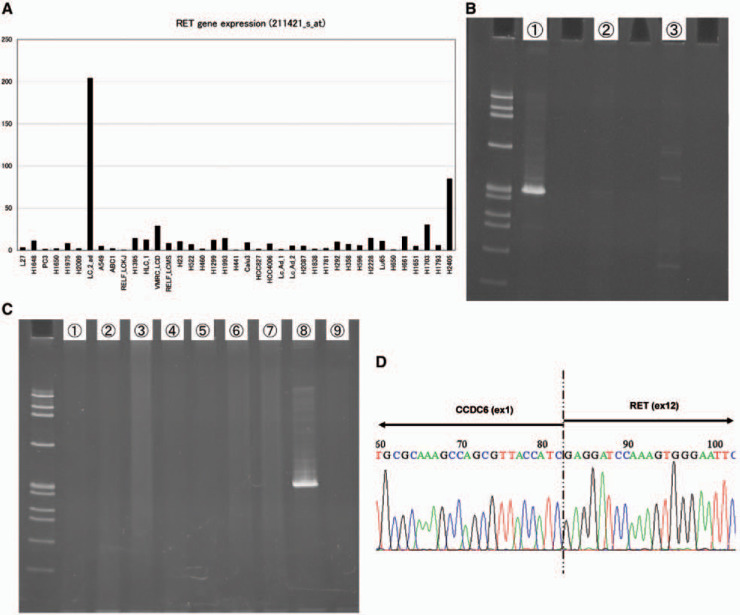 Fig. 1 Identification of CCDC6-RET fusion in LC-2/ad by fusion-specific RT-PCR. (Matsubara D, et al., 2012)
Fig. 1 Identification of CCDC6-RET fusion in LC-2/ad by fusion-specific RT-PCR. (Matsubara D, et al., 2012)
IFNγ and EGF Enhance the Expression of PD-L1 in LC-2/ad Cells
Immune checkpoint inhibitors targeting the programmed cell death‐1 (PD‐1)/PD‐1 ligand 1 (PD‐L1) axis have shown promising results in patients with non-small cell lung cancer (NSCLC). One major PD‐L1 inducer is IFNγ, which is secreted by T cells and NK cells. Importantly, IFNγ‐induced PD‐L1 is one of the major mechanisms by which cancer cells escape host immunity.
PD-L1 is up-regulated by both IFNγ and EGF in only LC-2/ad cells (Fig. 2), which led us to focus on the regulation mechanism of PD‐L1 expression in LC‐2/ad cells in the subsequent experiments. It was reported that IFNγ enhances PD‐L1 via the JAK-STAT pathway, while EGFR signaling regulates PD‐L1 expression via the PI3K‐AKT pathway. Next, the signaling pathway that regulates IFNγ‐ or EGF‐induced PD‐L1 expression was evaluated. RTK Ab array results showed that IFNγ activated the STAT1 pathway, while EGF activated AKT, p44/22 MAPK, and ribosomal protein S6 kinase (S6K) in LC‐2/ad cells.
Next, LC-2/ad cells were treated with several signal inhibitors targeting the candidate pathway to assess the blocking effect on EGF- or IFNγ-induced PD-L1 up-regulation. EGF-induced PD-L1 expression is blocked by the EGFR tyrosine kinase inhibitor, gefitinib, or signal inhibitor targeting PI3K (LY294002), MEK1 (PD98059), or S6K (PF4708671), which is the downstream pathway of EGFR as well as the JAK-STAT inhibitor, tofacitinib (Fig. 3a, b). However, IFNγ-induced PD-L1 expression is significantly blocked by tofacitinib, while the expression is relatively less but significantly affected by LY294002 and PD98059 in LC-2/ad cells (Fig. 3a, c). Basal expression of PD-L1 is down-regulated by LY294002, PD98059, and PF4708671, but no change is observed with tofacitinib (Fig. 3d). These findings suggest that EGF-induced PD-L1 expression is mainly regulated by the downstream pathway of EGFR via PI3K, MEK1, and S6K, while IFNγ-induced PD-L1 expression is mainly regulated by the JAK-STAT pathway in LC-2/ad cells.
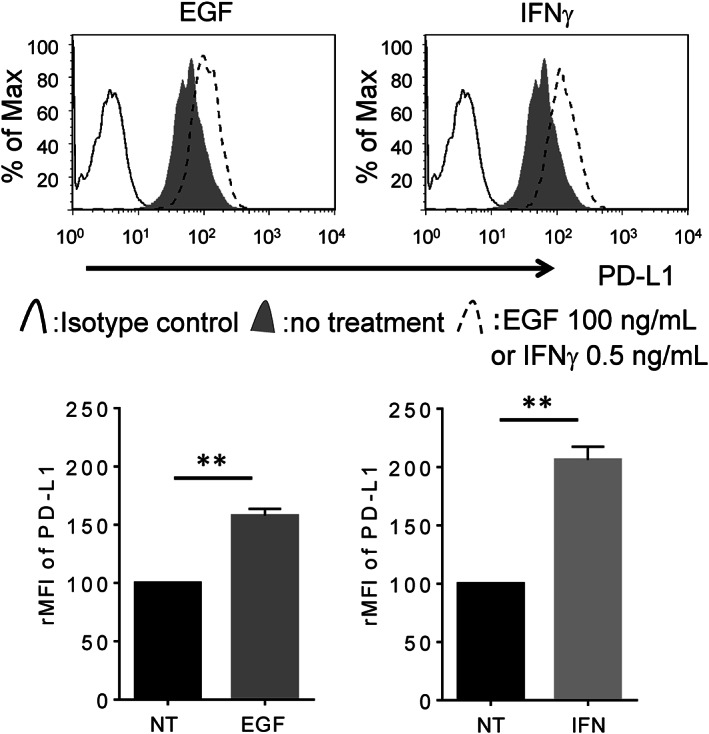 Fig. 2 PD‐L1 overexpression is induced by both EGF and IFNγ in LC‐2/ad cells. (Okita R, et al., 2021)
Fig. 2 PD‐L1 overexpression is induced by both EGF and IFNγ in LC‐2/ad cells. (Okita R, et al., 2021)
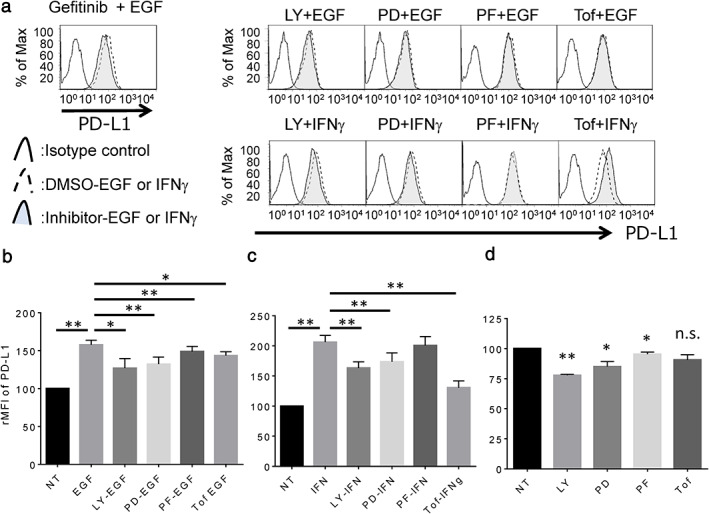 Fig. 3 Expression of PD‐L1 in LC‐2/ad cells treated with IFNγ or EGF followed by each signal inhibitor. (Okita R, et al., 2021)
Fig. 3 Expression of PD‐L1 in LC‐2/ad cells treated with IFNγ or EGF followed by each signal inhibitor. (Okita R, et al., 2021)
Intracellular ROS production and the AMPK/Akt/mTOR signaling pathway may be involved in autophagy and angiogenesis during the recovery of the heat-denatured dermis. The Akt/mTOR signaling pathway is a well-known pathway involved in the regulation of autophagy.
Ask a Question
Average Rating: 4.0 | 1 Scientist has reviewed this product
Professional
These cells allowed me to identify potential biomarkers associated with prognosis and treatment response in lung cancer patients.
15 Apr 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need