LLC-MK2
Cat.No.: CSC-C9110W
Species: Macaca mulatta (Rhesus macaque)
Source: Kidney
Morphology: epithelial
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The LLC-MK2 cell line (also called Llc-Mk2, LLC-MK-2, LLCMK2, or Lilly Laboratories Cell-Monkey Kidney 2) was initially generated and created in the mid-1950s from the kidney tissue of six healthy adult rhesus monkeys. These cells are involved in kidney functions ranging from filtering and reabsorption of material to secretion in vivo. These cells could be grown stably and passed through the lab, and multiplied rapidly – two to three times a week.
There are a number of distinctive properties of the LLC-MK2 cell line, one of which is that it is susceptible to numerous viruses. They have been shown to be susceptible to viruses ranging from enteroviruses to rhinoviruses, mucoviruses and the poxviruses, making them useful for virologic research. In addition, the LLC-MK2 cell line has been used to produce mumps vaccines and isolate parainfluenza virus. Therefore, applications of LLC-MK2 cell lines in scientific research include virus culture and study, vaccine production, drug screening, and gene function studies. They have also been used to study viral infection and replication mechanisms, and to evaluate the effects of antiviral drugs.
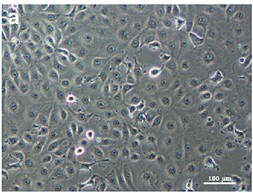 Fig. 1. Monolayer of uninfected LLC-MK2 cells (Chompoosri J, Thavara U, et al., 2016).
Fig. 1. Monolayer of uninfected LLC-MK2 cells (Chompoosri J, Thavara U, et al., 2016).
Treatment with Melatonin Induces a Reduction of Toxoplasma gondii Development in LLC-MK2 Cells
Toxoplasmosis, transmitted by Toxoplasma gondii, infects one in three of the world's population and is particularly toxic to the immune system of immunocompromised patients and pregnant women when not manifesting symptoms. The preventive medications pyrimethamine and sulfadiazine are already toxic, and alternatives are needed. The antioxidant and immunomodulator melatonin worked against parasitic infections, but little is known about T. gondii. Machado et al. tested melatonin's effect on T. gondii-infected monkey kidney LLC-MK2 cells to determine whether it was an alternative toxoplasmosis drug.
Cell viability did not decrease under melatonin treatment, except in highly concentrated amounts over 48 hours and 6 days (Fig. 1A). Control cells were time-dependent parasite growers (Fig. 1A), while the cells that were treated with melatonin had lower infection rates, which dropped substantially at 6 days (Fig. 1B). The IC50 of melatonin was 3 mM at 24 hours, 1.69 mM at 48 hours, and 1.13 mM at 6 days. Reversal of inhibition occurred when melatonin was removed after 24 hours, but not after 48 hours and 6 days (Fig. 1B). Untreated cells exhibited rosacea structures after 24h of incubation (Fig. 2a), multiple parasites 48h (Fig. 2b), and ruptured cells releasing parasites at 6 days (Fig. 2c). During 24h, melatonin greatly reduced parasite production (Fig. 2d), 48h (Fig. 3e), and 6 days (Fig. 3f), while retaining cell integrity (Fig. 2d–f). Parasites grew back but collapsed structurally upon 24h melatonin deprivation (Fig. 2g). Cells treated for 48h (Fig. 2h) and 6 days (Fig. 2i) had large vesicles but no parasite structures following withdrawal.
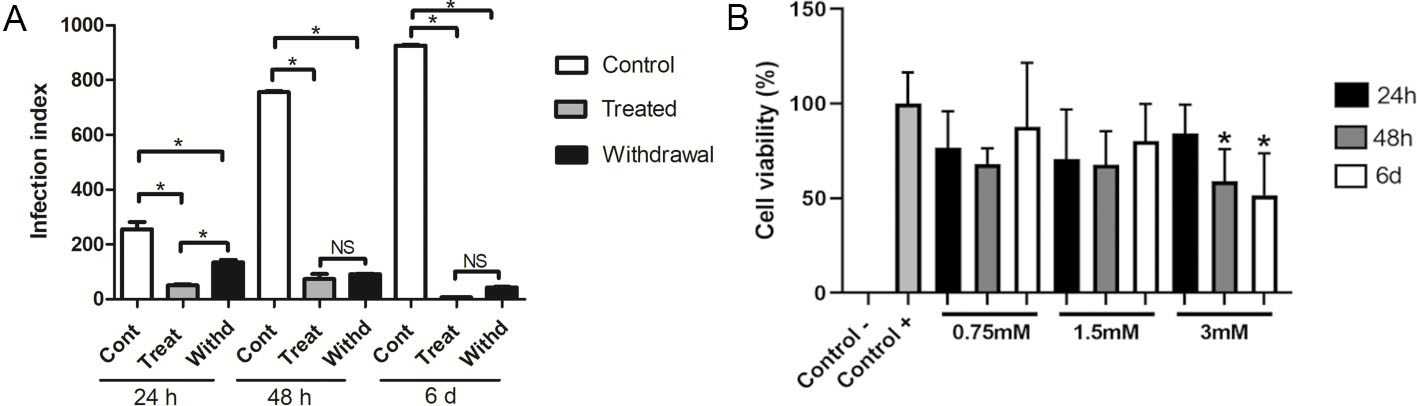 Fig. 1. (A) Cell viability of LLC-MK2 cells after melatonin treatment. (B) Infection index of Toxoplasma gondii in LLC-MK2 cells (Machado, N. I., Santos, T. A. T. D, et al., 2020).
Fig. 1. (A) Cell viability of LLC-MK2 cells after melatonin treatment. (B) Infection index of Toxoplasma gondii in LLC-MK2 cells (Machado, N. I., Santos, T. A. T. D, et al., 2020).
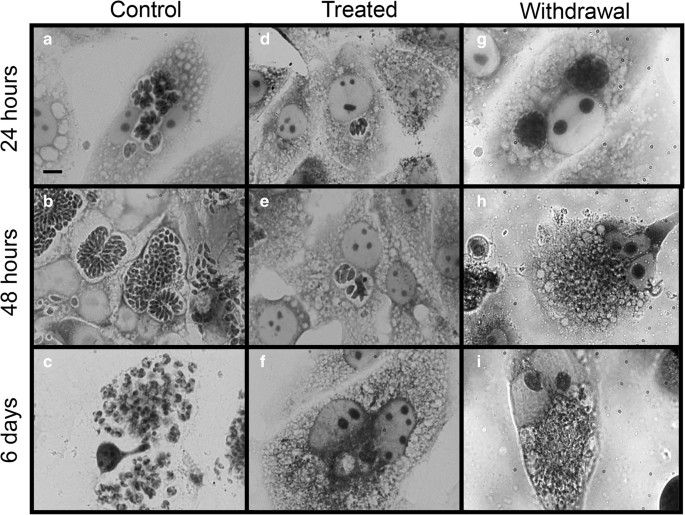 Fig. 2. Bright-field light microscopy images of LLC-MK2 cells infected with Toxoplasma gondii (Machado, N. I., Santos, T. A. T. D, et al., 2020).
Fig. 2. Bright-field light microscopy images of LLC-MK2 cells infected with Toxoplasma gondii (Machado, N. I., Santos, T. A. T. D, et al., 2020).
Depletion of mDia1 Improves Influenza A/NWS/33 Replication and Enhances Cytoskeletal Dynamics in LLC-MK2 Cells
Viruses rely on host cells for replication, and the cytoskeleton is a prime target for intraviral transmission and infection. More specifically, stabilising microfilaments and microtubules block human influenza A virus (H1N1) from entering LLC-MK2 cells through modulating cytoskeletal dynamics. The mammalian Diaphanous-related formin-1 (mDia1) appears to govern these processes and also to participate in actin fibrillogenesis and the assembly of microtubules. Yet few studies examine how mDia1 plays a role in viral infection. Conto team's work here explored how mammalian Diaphanous-related formin-1 (mDia1) controls cytoskeletal activity in response to influenza A virus infection.
They found that the formin protein mDia1 influences the cytoskeletal response during influenza A infection, with mDia1 partially co-localizing with actin and tubulin in infected cells. Therefore, they subsequently explored whether mDia1 contributes in some way to the development of the infection. To this end, they analyzed the impact of mDia1 depletion on A/NWS/33 virus replication in LLC-MK2 cells. mDia1 was depleted using RNA interference. As seen in Fig. 3a, knockdown efficacy was moderate at 24 h and more pronounced at 48 h, thus the latter was used for experiments. LLC-MK2 cells with depleted mDia1 were infected with A/NWS/33 virus for 24 hours. IIF analyses depicted a nearly doubled level of viral NP in mDia1-depleted cells (Fig. 3d, e), compared to control cells treated with control siRNA (Fig. 3b vs. c). TCID50 assays further revealed that mDia1 depletion significantly increased the yield of infectious viral progeny (P < 0.05, Fig. 3f). In further experiments, they explored how mDia1 depletion affects cytoskeletal stability. Reduced levels of acetylated alpha-tubulin were observed, enhancing MT dynamics (Fig. 4a). mDia1-depleted cells also exhibited decreased levels of actin and beta-tubulin and partial loss of filamentous actin, especially at the cell cortex (Fig. 4b1 vs. b, c1 vs. c, d vs. d1). The distribution of beta- and acetylated alpha-tubulin expanded across the cytoplasm, losing typical perinuclear localization (Fig. 4c1 vs. c, d1 vs. d).
 Fig. 3. The siRNA-mediated knockdown of mDia1 improves the outcome of influenza A/NWS/33 virus infection in LLC-MK2 cells (Conto D F, Fazzi A, et al., 2018).
Fig. 3. The siRNA-mediated knockdown of mDia1 improves the outcome of influenza A/NWS/33 virus infection in LLC-MK2 cells (Conto D F, Fazzi A, et al., 2018).
 Fig. 4. The siRNA-mediated knockdown of mDia1 improves MF and MT dynamics in LLC-MK2 cells (Conto D F, Fazzi A, et al., 2018).
Fig. 4. The siRNA-mediated knockdown of mDia1 improves MF and MT dynamics in LLC-MK2 cells (Conto D F, Fazzi A, et al., 2018).
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells