What Is the Role of Macrophage-Derived Exosomes in Gastric Cancer Progression?
Frontiers in Immunology. 2024 Feb 22; 15: 1327281.
Authors: Qiu Y, Lu G, Li N, Hu Y, Tan H, Jiang C
INTRODUCTION
As a key constituent of the tumor microenvironment (TME), macrophages frequently exhibit characteristics associated with an M2-like phenotype and collaborate with cancer cells to promote tumorigenesis, tumor progression, and resistance to therapy. The influence exerted by macrophages on the therapeutic response of cancer cells has garnered attention, leading researchers to explore their potential as targets for anticancer treatment. However, precise details regarding the intricate interactions between anticancer therapies and tumor-associated macrophages (TAMs) remain largely unknown.
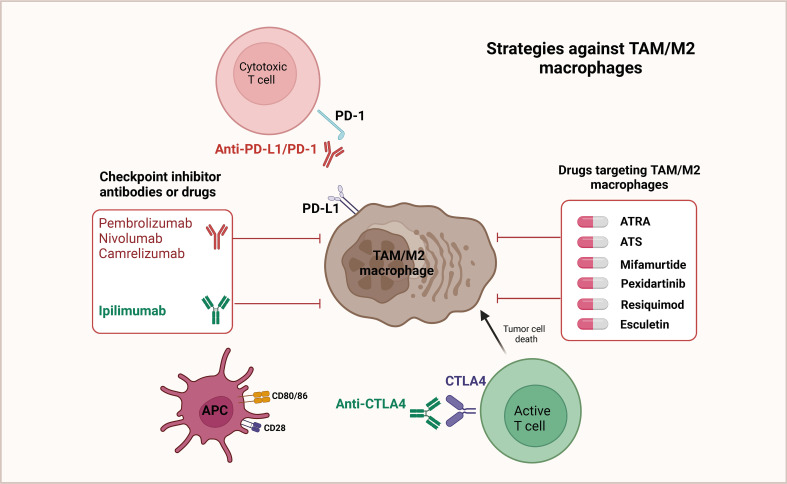 Fig. 1 Targeted therapeutic strategies against TAM/M2 macrophages.
Fig. 1 Targeted therapeutic strategies against TAM/M2 macrophages.
Regulating Cancer Proliferation, Migration, and Invasion
TAMs can manage the growth and metastasis of gastric cancer (GC) cells through exosomes and hereat encourage malignant tumor progression. TAMs were enriched in GCs and could be converted to M2 polarization to foster the migration of GC cells. Otherwise, apolipoprotein E (ApoE) was also increased in M2 macrophages and could penetrate GC cells, activating the PI3K-Akt signaling pathway in recipient GC cells to remodel the cytoskeleton to support migration. Treatment of GC cells with TAM-exos notably promoted cell invasion and migration, while treatment with JPYZ markedly inhibited the expression of miR-513b-5p in TAM-exos. Mechanistic experiments indicated that miR-513b-5p could target and inhibit PTEN and then activate the AKT/mTOR signaling pathway, thus encouraging GC invasion and metastasis in vivo and in vitro.
Table 1. Potential roles and mechanisms of TAM-derived exosomes in cell proliferation and migration of GC.
| Molecular | Parent cell/source | Target cell | Target | Biological function |
| ApoE | TAMs | GC cells | ApoE/PI3K-Akt | Promote GC metastasis |
| miR-513b-5p | TAMs | GC cells | miR-513b-5p/PTEN/AKT/mTOR | Promote GC invasion and metastasis |
| miR-487a | TAMs | GC cells | miR-487a/TIA1 | Promote cell proliferation and tumorigenesis |
| miR-21 | TAMs | GC cells | miR-21/PDCD4 | Promote GC metastasis |
Generating Drug Resistance
Drug resistance is one of the most important challenges in tumor therapy, which involves the resistance of tumor cells to the drug, consequently impairing the therapeutic effect and even causing treatment failure. This complex process includes multiple elements such as genetic mutations, epigenetic alterations, signaling pathway abnormalities, and the tumor microenvironment.
- Exosomes from M2 macrophages (M2-exos) facilitated DDP resistance in GC cells, and miR-21 grew in exosomes and cell lysates isolated from M2-polarized macrophages. Mechanistic experiments implied that macrophages could send miR-21 to GC cells through exosomes, which could downregulate the PTEN-activated PI3K/AKT signaling pathway to inhibit apoptosis, incurring the development of DDP resistance in GCs.
- M2-polarized macrophages were considered to send exosomes of miR-588 to GC cells and strengthen the resistance of GC cells to DDP, and overexpression of miR-588 improved the growth of DDP-resistant GC cells. Mechanistic experiments indicated that miR-588 could encourage the proliferation and apoptosis of DDP-exposed GC cells by targeting CYLD.
Table 2. Potential roles and mechanisms of TAM-derived exosomes in drug resistance of GC.
| Molecular | Parent cell/source | Target cell | Target | Biological function |
| miR-21 | TAMs | GC cells | miR-2/PTEN/PI3K/AKT | Inhibit cell apoptosis and induce DDP resistance |
| miR-223 | TAMs | GC cells | miR-223/FBXW7 | Promote doxorubicin resistance |
| miR-588 | TAMs | GC cells | miR-588/CYLD | Promote cell growth and induce DDP resistance |
| CRNDE | TAMs | GC cells | CRNDE/NEDD4-1/PTEN | Promote cell growth and induce CDDP resistance |
| circ-0008253 | TAMs | GC cells | circ-0008253/ABCG2 | Promote cell viability, and tumor size, and reduce sensitivity to OXA |
Promoting Immune Escape of Tumor Cells
Tumor immune escape is a process in which tumor cells evade the surveillance of the immune system, suppress the anti-tumor immune response, and enhance the proliferation, invasion, and metastasis of tumor cells. The elements affecting tumor immune escape are composed of the tumor cells themselves and the tumor-induced immunosuppressive microenvironment.
miR-16-5p grew in M1 macrophage exosomes, was sent to GC cells, and inhibited tumor formation in vitro and in vivo by detecting PD-L1. Also, exosomal miR-16-5p triggers T-cell immune responses and thus inhibits GC progression. M2-GC cells can absorb and internalize exos, promoting cell proliferation and migration and inhibiting apoptosis. The consequences of mechanistic experiments demonstrated that M2-Exos could improve the phosphorylation of P38 and the expression of programmed death ligand 1 (PD-L1) and therefore help GC progression, achieving immune escape through the increase in the expression of PD-L1.
Table 3. Exosome-mediated immune escape of tumor cells.
| Molecular | Secreted cells | Recipient cells | Target | Role and mechanism in immune suppression |
| miR-1246 | Colon cancer | Macrophages | TGF-β signaling | miR-1246-expressing TAMs have enhanced TGF-β signaling, which increases the Treg population in mouse tumors and promotes immune suppression. |
| miR-23a-3p | Hepatocellular carcinoma | Macrophages | Akt-PDL1 pathway | miR-23a-3p inhibits PTEN and induces PDL1 expression, which decreases the CD8+ T-cell ratio and promotes T-cell apoptosis. |
| miR-208b | Colorectal cancer | T cells | Cell death factor 4 (PDCD4) | PDCD4 promotes CD4+ Treg expansion, which promotes CRC growth and oxaliplatin resistance. |
| miR-107 | Gastric cancer | MDSC | DICER | miR-107 targets 3'UTRs of DICER and PTEN in MDSCs. DICER downregulation promotes MDSC expansion, whereas PTEN inhibition upregulates the PI3Kinase pathway and promotes proliferation. |
| TUC339 | Hepatocellular carcinoma | THP1 monocytes | IL-1β, TNF-α, CD86 | TUC339 overexpression decreases the production of proinflammatory cytokines IL-1β and TNF-α, T-cell activator CD86 expression, and phagocytic activity in THP1 cells. |
| RPPH1 | Colorectal cancer | Macrophages | RPPH1 | RPPH1 increases the expression of M2 macrophage markers CCL17, CCL18, CXCL8, IL-10, and TGF-β. M2-polarized macrophages promote CRC proliferation and metastasis. |
| CRNDE-h | Colorectal cancer | CD4+ T cells | RORγT | RORγT binds to IL-17 promotor and triggers CD4+ T-cell differentiation into immunosuppressive IL-17-producing Th17 cells. |
Creative Bioarray Relevant Recommendations
| Service/Product Types | Description |
| Macrophages | We offer a wide range of macrophages. According to their polarization status, macrophages can be divided into M0, M1 (classically activated or pro-inflammatory), and M2 (alternatively activated or anti-inflammatory) macrophages. |
| Gastric Tumor Cells | Our human tumor cells have the original pathological diagnosis and are analyzed for key mutations, presenting the real characteristics of their in vivo state and maintaining heterogeneity across multiple passages, enabling you to expedite your drug discovery and process development. |
| Exosome Research Services | Creative Bioarray provides a complete set of services for exosome research and resolves your issues quickly and efficiently. We also offer comprehensive support for customized services to meet your needs. |
RELATED PRODUCTS & SERVICES
Reference
- Qiu Y, et al. (2024). "Exosome-mediated communication between gastric cancer cells and macrophages: implications for tumor microenvironment." Front Immunol. 15: 1327281.