What Human Disease Models Are Available for Drug Development?
In the field of drug development, the availability of reliable human disease models plays a pivotal role in understanding disease mechanisms, evaluating drug efficacy, and predicting potential toxicities. Over the years, significant advancements have been made in developing a diverse range of disease models that closely mimic human physiology and pathology.
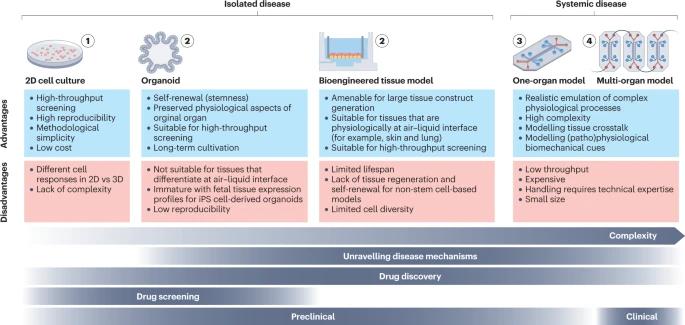 Fig. 1 Overview of different disease models. (Mashouri L, et al., 2019)
Fig. 1 Overview of different disease models. (Mashouri L, et al., 2019)
2D Cell Cultures
Two-dimensional (2D) cultured patient-derived cells are an invaluable tool for studying disease phenotypes and mechanisms, especially in the early stages of drug development. Primary cells are the preferred option due to their higher genetic heterogeneity compared to cell lines, but their limited availability or in vitro proliferation capacity limits their use. Multipotent adult stem cells (ASCs) and induced pluripotent stem cells (iPS cells) can help overcome this limitation, as they can be easily and indefinitely expanded and transformed into any somatic cell. Engineered cells, such as reporter cell lines, are also commonly used because they are amenable to high-throughput manufacturing and have high reproducibility and lower costs compared to stem cell-based methods.
- One of the key advantages of 2D cell cultures is their ease of use and cost-effectiveness. They are relatively simple to set up and maintain, requiring minimal specialized equipment. This makes them accessible to a wide range of researchers and allows for high-throughput screening of compounds and drugs.
- 2D cell cultures also offer the advantage of scalability, allowing large numbers of cells to be cultured simultaneously. This enables researchers to perform experiments in a high-throughput manner, facilitating screening of potential drug candidates and evaluation of their effects on cell viability, proliferation, and other cellular processes.
- However, it is important to note that 2D cell cultures have inherent limitations. They do not fully recapitulate the complex architecture and physiological environment of native tissues and organs in the human body. The flat nature of the culture surface restricts cell-cell interactions and limits the formation of three-dimensional structures seen in vivo. Additionally, the absence of the extracellular matrix, which provides structural support and biochemical cues in native tissues, can influence cellular behavior and responses.
Bioengineered Tissue Models
To overcome the limitations of 2D cell cultures, bioengineered tissue models have emerged as a promising alternative. Bioengineered tissue models are primarily generated from human stem cells or primary cells, the latter of which are isolated from surgically excised human tissue or non-transplantable organs.
By leveraging tissue engineering techniques, researchers have successfully developed bioengineered tissue models that closely resemble human organs, such as the liver, heart, lung, and kidney. These models provide a more realistic platform for studying disease progression, drug metabolism, and toxicity, thereby enhancing the predictability of preclinical studies.
Organoids
Organoids are self-organizing 3D structures generated from tissue-specific ASCs or iPS cells. Stem cells are embedded in an extracellular matrix that provides the scaffold for tissue growth. Organoid formation is then guided by a cocktail of growth factors that are pivotal for tissue development in vivo, which enables stem cells to maintain their differentiation and self-renewal capacity.
Protocols for the generation of iPS cell-derived organoids are available for a variety of human organs, including the gut, stomach, liver, pancreas, lung, thyroid, kidney, retinal and optic cup, and brain. These miniaturized organs offer unprecedented opportunities to study human development, model diseases, and test therapeutic interventions.
Organs-on-Chips
Organs-on-chips represent a cutting-edge technology that combines microfluidics, living cells, and mechanical forces to recreate the microenvironment and physiological functions of human organs in vitro. These miniature devices consist of cell-lined channels that mimic the structural and functional characteristics of specific organs, allowing for the study of disease mechanisms and drug responses under more physiologically relevant conditions. These micro-physiological systems enable dynamic monitoring of cellular responses, drug transport, and organ-organ interactions, thereby bridging the gap between traditional in vitro models and human clinical trials.
Creative Bioarray Relevant Recommendations
Creative Bioarray offers many types of cells for drug development. Additionally, we offer comprehensive pharmacology and pharmacodynamics, toxicology, safety evaluation, pharmacokinetics, and toxicokinetics services.
| Product/Service Types | Description |
| Primary Cells | The team of cell culture experts at Creative Bioarray provides you with a large and unique collection of high-purity, low-passage human and animal primary cells. Our primary cells cover a wide range of cell types (epithelial cells, endothelial cells, smooth muscle cells, and many other key types) from a large variety of tissues (skin, liver, bone, brain, and more) in both cryopreserved and proliferating formats. |
| Stem Cell Research | Whether you want to obtain and expand pluripotent cells, differentiate them along specific lineages, or edit your cells, our products and services support your goals. |
| In Vitro DMPK Services | Creative Bioarray provides a variety of in vitro ADME/PK services, including high-throughput ADME screening, in vitro binding, in vitro metabolism, in vitro permeability, and transporter assays. |
| In Vivo DMPK Services | Creative Bioarray provides in vivo drug metabolism and pharmacokinetic (DMPK) services to support your drug development studies of in vivo absorption, distribution, metabolism, and excretion of drug candidates. Our in vivo DMPK services cover a comprehensive range of different animal studies in several species. |
Reference
- Loewa A, et al. (2023). "Human disease models in drug development." Nat Rev Bioeng. 11, 1-15.

