Tips for Choosing the Right Protease Inhibitor
Protease inhibitors are essential tools in biological research and clinical applications, used to prevent the activity of proteases-enzymes that degrade proteins. Selecting the right protease inhibitor is critical for the success of experiments and therapeutic interventions.
What Are Protease Inhibitors?
Protease inhibitors are molecules that impede the function of proteases-enzymes responsible for breaking down proteins and peptides. These inhibitors can be natural or synthetic and are vital in a range of biological processes, including cell signaling, immune responses, and the maintenance of homeostasis. They are particularly significant in fields such as cancer research, where they can inhibit the activity of proteases that contribute to tumor growth and metastasis, as well as in virology, where they are essential for targeting viral proteases.
Some of the most common protease inhibitors used in the laboratory include AEBSF (4-(2-Aminoethyl)-benzenesulfonylfluoride hydrochloride), aprotinin, bestatin, EDTA/EGTA, leupeptin, pepstatin A and PMSF (Phenylmethanesulfonyl fluoride). Among these inhibitors, AEBSF and PMSF are among the most widely used in the lab. These compounds gained popularity since they do a great job of inhibiting the action of several serine proteases (e.g. trypsin, chymotrypsin, and thrombin), which happens to be the most predominant protease group found in cell and tissue samples.
Classification of Protease Inhibitors
Protease inhibitors can be classified based on their specific targets or the types of proteases they inhibit:
| Types of protease inhibitors | Details |
| Serine protease inhibitors (Serpins) | These are among the most well-studied inhibitors and play crucial roles in regulating blood coagulation and inflammation. |
| Cysteine protease inhibitors | These target cysteine proteases, which are involved in processes like apoptosis and immune system modulation. |
| Aspartic protease inhibitors | Notably important in inhibiting viruses, these inhibitors are vital in treatments for conditions like HIV. |
Protease inhibitors can be categorized based on their origins and chemical structures:
| Types of protease inhibitors | Details |
| Natural inhibitors | These are often produced by organisms as a defense mechanism against pathogens. For instance, slit peptides and serpins are vital in regulating protease activity in various biological processes. |
| Synthetic inhibitors | Designed through medicinal chemistry, these inhibitors are tailored for enhanced efficacy and specificity against particular proteases. They often demonstrate improved stability and potency compared to natural variants. |
How Do Protease Inhibitors Work?
The primary function of protease inhibitors is to bind to the active sites of proteases, preventing substrate access and subsequent enzymatic activity. This binding can occur through various interactions, including hydrogen bonding, hydrophobic interactions, and covalent bonds, depending on the type of inhibitor. The functional mechanism of protease inhibitors can also involve allosteric modulation, where the inhibitor binds to an allosteric site, inducing a structural change in the protease that reduces its activity.
Binding mechanisms
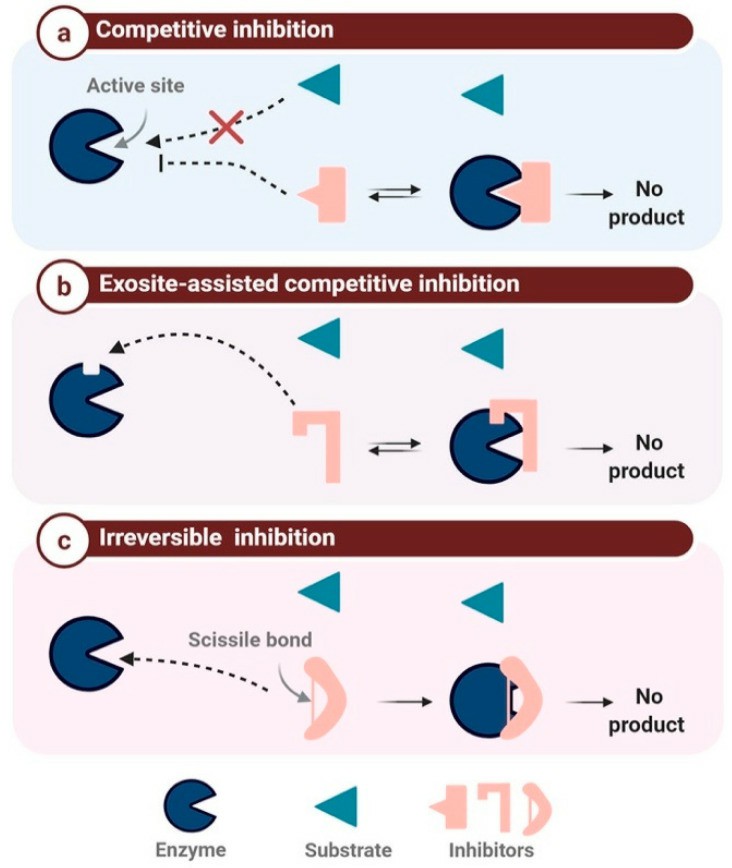 Fig.1 The mechanism-based classification of protease inhibitors. (Vargason AM, et al., 2021)
Fig.1 The mechanism-based classification of protease inhibitors. (Vargason AM, et al., 2021)
- Competitive inhibition. Inhibitors compete with the substrate for the active site of the protease. By occupying this site, they prevent substrate binding, effectively halting enzymatic activity.
- Non-competitive inhibition. These inhibitors bind to a site different from the active site. This binding causes a conformational change in the protease, diminishing its activity regardless of substrate presence.
- Allosteric modulation. Some inhibitors bind to allosteric sites, inducing structural changes that affect the enzyme's function. This can either enhance or inhibit the enzyme's activity, depending on the nature of the inhibitor.
Molecular mechanisms
- Hydrogen bonds. These weak bonds play a significant role in stabilizing the inhibitor-protease complex.
- Hydrophobic interactions. Non-polar regions of the inhibitor and protease can interact favorably, further stabilizing the complex.
- Covalent bonding. Some irreversible inhibitors form covalent bonds with the protease, permanently inactivating it. This type of inhibition is particularly common in certain enzyme-targeting drugs used in cancer and viral therapies.
How to Select the Right Protease Inhibitor?
Identify the target protease
Understanding the specific protease involved in your research or treatment is the first step in selection. Each protease has unique characteristics, and knowing its structure and function is essential. For example, if targeting a serine protease like trypsin, selecting a specific serine protease inhibitor would be critical for effective inhibition.
Assess inhibitor specificity
Inhibitor specificity is crucial to minimize off-target effects. High specificity reduces potential side effects and enhances the accuracy of experimental results. Tools like biochemical assays can help evaluate the specificity of different protease inhibitors.
Consider inhibitor potency
The potency of an inhibitor is measured by its ability to effectively reduce protease activity at low concentrations. This is typically quantified using IC50 values, which reflect the concentration required to inhibit 50% of the enzyme's activity. When selecting inhibitors, consider those with lower IC50 values, as they offer higher efficacy.
Evaluate formulation and stability
Stability under experimental conditions is another important aspect. Protease inhibitors can vary significantly in terms of heat stability and storage requirements. Those with a longer shelf-life and stability at room temperature are often preferable in laboratory settings.
Use a cocktail
A protease inhibitor cocktail is usually the first and most logical choice when lysing cells, homogenizing tissues, or performing any other protocol that compromises the cell membrane and triggers the release of proteases from the cellular compartments. By using a cocktail, you can better protect your protein from the action of a wide range of proteases without spending a lot of time and going through costly trial and error to find the right inhibitor. Protease inhibitor cocktails are also popular among scientists for their inherent reliability and reproducibility.
Creative Bioarray Relevant Recommendations
| Cat. No. | Product Name |
| FREA--14 | Protease |
| FREA--31 | Protease Buffer |
| CM-1015X | SuperCult® Trypsin-EDTA for Primary Cells |
Reference
- Jmel MA, et al. (2021). "Insights into the Role of Tick Salivary Protease Inhibitors during Ectoparasite-Host Crosstalk." Int J Mol Sci. 22 (2): 892.

