Stability of ADAs in Human Serum
Journal of Immunological Methods. 2024 Feb; 525: 113616.
Authors: Solberg H, Bartholdy C, Landsy LH, Andresen LO.
INTRODUCTION
The stability of anti-drug antibodies (ADAs) is important as ADA-analysis should be reliable over time at different storage conditions. The stability of anti-insulin antibodies in serum samples was assessed after short-term storage at different temperatures and after long-term storage at -20°C. Correlation between measurements was tested and acceptance criteria for incurred sample reanalysis were applied.
METHODS
For the short-term stability experiments twelve samples from type 1 and type 2 diabetic patients, containing medium or low amounts of anti-insulin antibodies, were acquired commercially.
- Short-term stability. The short-term stability of the anti-insulin antibodies in human serum was monitored by analysis of samples from 12 different subjects that all were positive for antibodies to insulin. The experiment performed was designed to cover short-term storage at 4°C and at 22°C, respectively. The short-term stability of anti-insulin antibodies at 22°C was examined for the variability between the measurements at 0 h and at 24 h, 48 h, and 72 h, respectively, by calculation of %difference. Analysis results for samples snap frozen on dry ice for 4 h, or at 4°C for 1 week, and 2 weeks, respectively. Similarly, to the analysis results for sample aliquots stored at 22°C, the results from sample aliquots snap frozen on dry ice for 4 h and at 4°C for 1 and 2 weeks, respectively, were subject to evaluation by their %difference
- Long-term stability. To document long-term stability and comparability of results throughout the study, 76 samples were re-analyzed after different periods and grouped in three storage periods A: 2.7-4.2 years (16 samples), B: 4.8-5.3 years (39 samples) and C: 6.0-6.3 years (21 samples). The effect of freeze-thaw events (FT) during long-term storage at -20°C was assessed by analyzing the %difference values calculated for each sample.
- Browse our recommendations
| Product/Service Types | Description |
| Chemical Stability Assays | Creative Bioarray provides chemical stability assays to determine the degradation of drugs under different conditions. These assays are carried out using various media, including simulated intestinal fluid (SIF), simulated gastric fluid (SGF), or buffers of different pH values. |
| Plasma Stability Assay | Creative Bioarray's plasma stability assay can measure the stability of compounds in plasma, helping customers find unstable compound structures and screen prodrugs. |
| Physicochemical Characterization Assays | Creative Bioarray provides physicochemical characterization assays to help customers accurately evaluate compounds' physicochemical properties such as solubility and stability, reducing drug development difficulties. |
| In Vitro DMPK Services | Creative Bioarray provides a variety of in vitro ADME/PK services, including high-throughput ADME screening, in vitro binding, in vitro metabolism, in vitro permeability, and transporter assays. |
RESULTS
- Results from the analysis of samples stored at 22°C are presented. There was very little variation between the results of the analyses performed after 24, 48, and 72 h, respectively. The %difference calculated for each measurement is depicted as a function of the measurement at 0 h. The acceptance criteria were that the %difference of 66.7% of the samples should be within ±30%. All re-analysis results, except one, were within the ±30% acceptance interval. The results outside the acceptance criteria for the %difference were from sample no.10 after 24 h (35.71%), corresponding to 8% (1 out of 12) samples. This sample had low %B/T-values at 0 h (2.8 %B/T) and would thus more likely have %difference-values outside the ±30% acceptance interval. At 4°C, the 12 samples showed only little variation between the baseline result at 0 h and the results after short-term storage. The evaluation by %difference showed that after 1 week, one out of 12 (8%) of the re-analyses results were outside the ±30% acceptance range.
The results of this study indicate that the anti-insulin antibodies in human serum are not affected by short-term bench top storage at about 22°C for up to at least 72 h or at storage at a refrigeration temperature of approximately 4°C for up to at least 2 weeks. Also, ADA stability was maintained by snap freezing, storage on dry ice for 4 h followed by storage at -20°C.
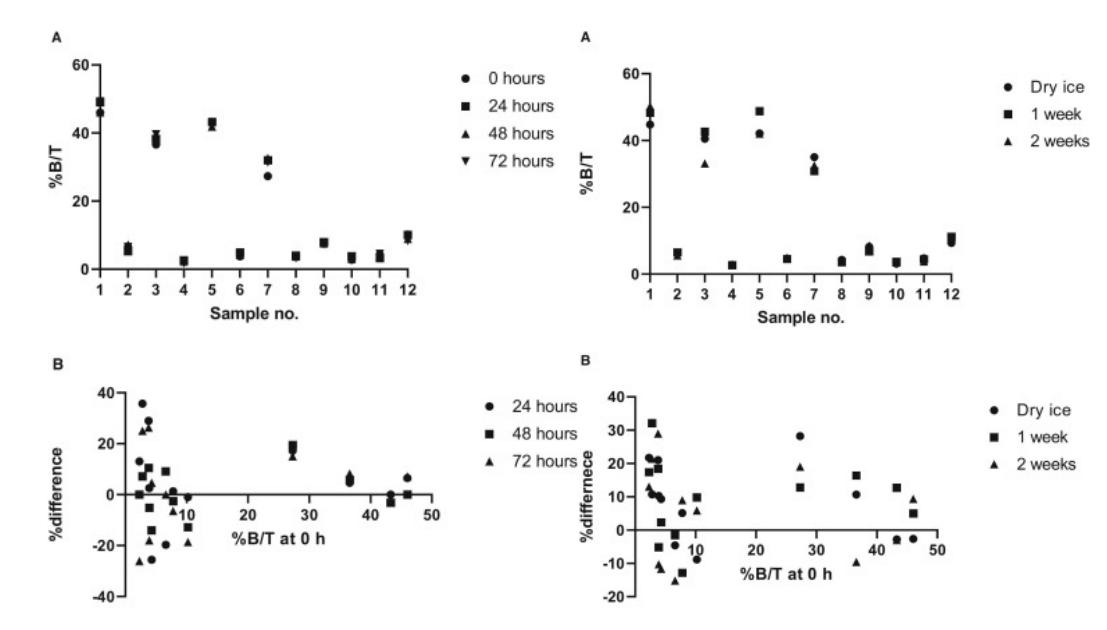 Fig. 1 Left: Short-term stability of anti-insulin antibodies at 22°C in 12 different serum samples; Right: Short-term stability of anti-insulin antibodies at 4°C in 12 different serum samples.
Fig. 1 Left: Short-term stability of anti-insulin antibodies at 22°C in 12 different serum samples; Right: Short-term stability of anti-insulin antibodies at 4°C in 12 different serum samples.
- Group A had 2 out of 16 (12.5%) samples, group B had 9 out of 39 (23.1%) samples and group C had 7 out of 21 (33.3%) samples with %difference-values outside the acceptance interval of ±30%. All three groups A, B, and C were within the ISR acceptance range of 66.7% samples having less than ±30% difference between the first and last measurements. Linear regression analyses with the original analysis results as reference were performed for all 76 samples in the long-term stability assessment. The fitted line of the linear regression analysis had a slope of 0.9167, Y-intercept at -3.8704, X-intercept at 3.548, and correlation coefficient of 0.9357 indicating a very strong correlation between data sets. This study indicates that the long-term storage does not affect the stability of anti-insulin antibodies in undiluted human serum samples after up to 6.3 years at -20°C. The box plot of the %difference values obtained with the long-term storage samples with intermediate FT indicates that the FT does not have any negative influence on the antibody stability as both the mean and median %difference values from the analyses of the samples are very close to zero. The results show that the stability of the antibodies was not affected after four FT during the storage time.
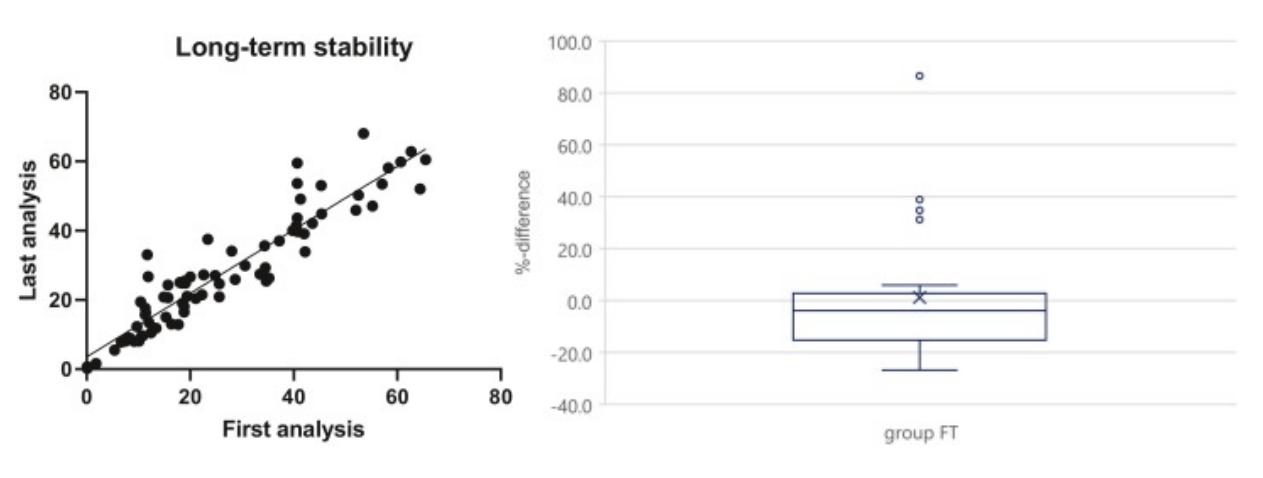 Fig. 2 Left: Linear regression of long-term stability data; Right: Long-term stability of anti-insulin antibodies after storage at −20°C with four FTs during storage.
Fig. 2 Left: Linear regression of long-term stability data; Right: Long-term stability of anti-insulin antibodies after storage at −20°C with four FTs during storage.
SUMMARY
ADAs were stable after 72 h at 22°C, after 2 weeks at 4°C, and after 6.3 years at -20°C. The study confirms that ADAs in serum are stable for several years at -20°C and suggests that investigation of short- and long-term stability of ADAs is not needed if samples are handled at standard laboratory conditions.
RELATED PRODUCTS & SERVICES
Reference
- Solberg H, et al. (2024). "Stability of anti-drug antibodies in human serum samples." J Immunol Methods. 525: 113616.